
Abstract
Aims: Coronary artery fistulae represent one of the most challenging anatomical defects to define accurately. We aimed to investigate the additional benefit conferred by volume rendering of tomographic images and 3D printing for diagnosis and interventional planning.
Methods and results: Four cases of coronary fistulae were considered for transcatheter closure. Multidetector computed tomography (three cases) or cardiac magnetic resonance (one case) images were acquired and segmented using Mimics software. Each case was reviewed after incremental consideration of diagnostic resources: two cardiologists reported source and volume-rendered images; device closure was discussed by the interventional cardiology team. All diagnoses and planned management were reviewed after inspection of a 3D model. Using source images alone, both cardiologists correctly described the course and drainage in two out of four cases. Aided by volume rendering, this improved to three out of four cases. Inspection of the 3D printed model prompted the planned interventional approach and device sizing to be altered in two out of four cases. In one out of four cases, the intervention was abandoned after inspection of the 3D printed model.
Conclusions: Diagnosis and management of patients with coronary artery fistulae rely on detailed image analyses. 3D models add value when determining the feasibility of, and the approach to intervention in these cases.
Abbreviations
3D: three-dimensional
AV: atrioventricular
CHD: congenital heart disease
CMR: cardiac magnetic resonance
LAA: left atrial appendage
LV: left ventricle
MDCT: multidetector computed tomography
RA: right atrium
RCA: right coronary artery
STL: stereolithography
SVC: superior vena cava
TOE: transoesophageal echocardiography
TTE: transthoracic echocardiography
VR: volume-rendered
Introduction
Additive manufacturing offers advantages for diagnosis, decision making and treatment planning in structurally complex congenital heart disease (CHD).
The purpose of this study was to evaluate the incremental contributions of conventional reporting of cardiac magnetic resonance (CMR) and computed tomography (CT) images, three-dimensional (3D) volume rendering, and 3D printed models, to the diagnosis and interventional planning of coronary artery fistulae.
Materials and methods
Three adults and one child with coronary artery fistulae were referred to our centre for a second opinion or intervention. Relevant anatomy (Figure 1) was segmented from multidetector computed tomography (MDCT) or 3D balanced steady-state free precession (bSSFP) images using Mimics medical software and Materialise 3-matic (version 10.0 & 18.0, respectively; Materialise NV, Leuven, Belgium). Intensity thresholding, cropping and region growing tools were used for segmentation and refined using manual editing (approximate mean segmentation time: three hours). A thickness of 2 mm was introduced outside the surface to yield a hollow model maintaining the image-derived blood pool wall. Generated stereolithography (STL) files were sent to the polyjet printer (Objet500 Connex1; Stratasys, Ltd, Eden Prairie, MN, USA), printing in flexible TangoPlus FullCure® 930 (Stratasys) for interventional simulation (Figure 2).
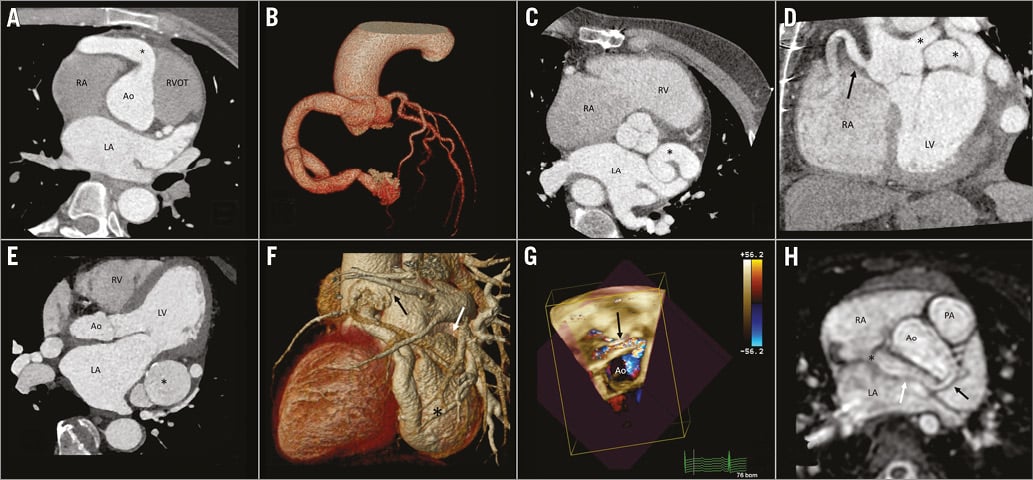
Figure 1. Reformatted images of MDCT and CMR. A) & B) Patient 1. A) RCA origin (*). B) Volume-rendered image of the fistula. C) & D) Patient 2. Origin and course of the left (*) and right (arrow) coronary fistulae in modified axial (C) and coronal views (D). E) & F) Patient 3. E) Large aneurysm sac (*) and course of the fistula, axial view (note calcification). F) VR image of the fistula showing large aneurysm sac (*), second aneurysm (white arrow) and drainage towards the LA (black arrow). G) & H) Patient 4. G) TTE showing course of the fistula around the aortic root (*). H) Origin of the fistula (black arrow), course (white arrow) and drainage (*) on a modified axial view from 3D-bSSFP.
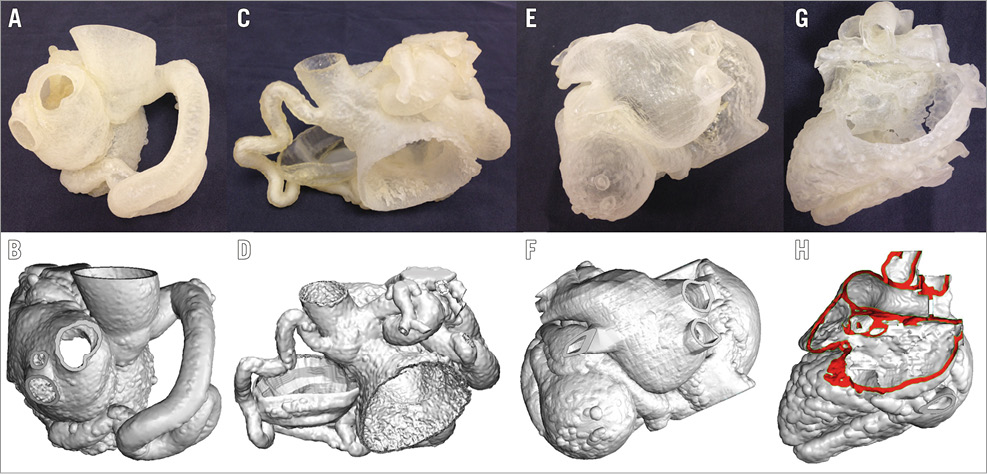
Figure 2. Patient-specific surface-rendered image and 3D model for patient 1 (A & B), 2 (C & D), 3 (E & F), 4 (G & H).
For the paediatric case, due to tearing of the TangoPlus model during simulation, a second model was fabricated in a flexible thermoplastic polyurethane by fused deposition modelling (Witbox printer; BQ, Las Rozas de Madrid, Spain).
REVIEW OF DIAGNOSTIC INFORMATION
The three sources of diagnostic information (raw images, volume-rendered [VR] images and 3D model) were reviewed incrementally to assess their value.
Two cardiologists (each having >10 years’ experience of CHD imaging) reviewed the data independently, reporting origin, course and drainage of fistulae structures and indicating whether they could represent the findings verbally.
Source and VR images were used to determine the best way to proceed regarding interventional decision making at a multidisciplinary meeting. The 3D model was subsequently shown to re-evaluate the plan.
We assessed the incremental value of each resource by comparing the reporting at each stage and variations in the advice given.
Results
Case 1. (41-year-old female). Both observers correctly identified a giant right coronary artery (RCA) to left ventricle (LV) fistula (Figure 1A, Figure 1B) from source MDCT data.
Case 2. (59-year-old male). From source CT data, both correctly reported a fistula involving both coronary arteries with dilation along their entire, respective lengths and their drainage to the coronary sinus (CS) (Figure 1C, Figure 1D). However, the complexity of the courses precluded verbal communication without the aid of VR reconstruction.
Case 3. (67-year-old male). The patient exhibited a fistulous network of the left coronary artery (LCA), with two aneurysms in series draining to the left atrial appendage (LAA) and the CS (Figure 1E, Figure 1F). Both observers reported the origin and CS drainage from source MDCT data. One observer failed to mention the drainage of the second aneurysm to the LAA. This error was corrected when analysing segmentation prepared for 3D printing (Figure 2F).
Case 4. (2-year-old boy). The fistula originated from the left main stem and drained into the superior vena cava-right atrium (SVC-RA) junction surrounding the aortic root. CMR, transoesophageal echocardiography (TOE) and transthoracic echocardiography (TTE) were performed (Figure 1G, Figure 1H, Moving image 1). Both observers correctly reported from source images, with no incremental benefit from volume rendering or 3D printed models.
CONTRIBUTION TO DECISION MAKING AND PLANNING
Interventional cardiologists inspected the 3D model and reconsidered decisions initially made with source and VR images. For those referred for cardiac catheter intervention, simulation of the proposed procedure was performed using the 3D model in the cathlab.
Case 1. In vitro simulation (Figure 2A derived from rendered segmentation Figure 2B) allowed planning of the best landing point for the occlusion device (Moving image 2). During the procedure, selective coronary angiography confirmed anatomy. As planned, closure was achieved with a 16 mm AMPLATZER™ Vascular Plug II (AVP II; St. Jude Medical, St. Paul, MN, USA) positioned near the drainage of the fistula to the LV.
Case 2. After multidisciplinary discussion, the team endorsed catheter intervention. However, interventional cardiologists only realised the extent of the involvement of both coronary arteries after inspecting the model (Figure 2C derived from rendered segmentation Figure 2D). Hence, its review changed the decision, leading to conservative management.
Case 3. After reviewing the imaging data, the team decided that the complexity of the anatomy would result in a high-risk procedure. Review of the 3D model (Figure 2E derived from rendered segmentation Figure 2F) did not change the decision to advise surgery over transcatheter intervention. Despite this, after discussion with the patient, percutaneous intervention was planned and performed at the referring centre.
During the procedure, the first device (a 16 mm AVP II), aiming to close the connection to the LAA, embolised into the large aneurysm sac. Closure was achieved with a 16 mm VSD Occluder (St. Jude Medical). A 14 mm AVP II was released at the drainage to the CS. A 3×18 mm Endeavor® drug-eluting stent (Medtronic, Minneapolis, MN, USA) was placed in the left circumflex to avoid its occlusion and subsequent ischaemia in relation to the device closing drainage to the LAA.
Case 4. The 3D model aided the selection of the approach and device (Figure 2G derived from rendered segmentation Figure 2H). During simulation, the plug was placed at the SVC-RA junction, closing the final portion of the fistula and its drainage into the atrium. During the intervention, closure was achieved using an 8 mm AVP II plug placed at the mid-point of the fistulous course (Moving image 3). Distal placement of the plug, as planned, failed in the patient due to the delivery sheath displacement back to the RA.
Active reference to models during the intervention in cases 1 and 4 aided spatial orientation throughout the procedure.
Discussion
Methods for 3D visualisation of the cardiac anatomy (such as MDCT and CMR) are vital for patients with complex CHD. We have previously published our experience on the utility of dual-phase whole-heart imaging for coronary visualisation in CHD1,2.For the imaging expert, our findings suggest that VR and 3D printing contribute little to diagnosis in cases of simple coronary artery fistula. However, where the anatomy is complicated, VR and 3D models aid the interpretation of these images and facilitate communication of the morphology to the interventional team.
Our study demonstrates the importance of a physical 3D representation of the anatomy in complex cases, particularly when planning the approach and the device for closure. Simulation may ensure that the correct equipment is available and hence reduce procedure times. This is in line with previous reports of interventional planning in CHD3.
In keeping with a previous systematic review4, we relied on manual editing and semi-automated methods of image segmentation. This resulted in a long processing timeline, inconsistent with routine clinical practice.
Limitations
In addition to the image segmentation time, there are also limitations of the model simulation. As highlighted by case 4, the 3D printed model could not reliably replicate the mechanical properties of the heart’s tissues and did not simulate blood flow.
Conclusions
Volume rendering of MDCT and CMR images is essential for accurate diagnosis in complex coronary artery fistulae. 3D printed models are helpful for simulation and preprocedural planning. They represent a valuable communication tool, enabling the imaging specialist to convey their understanding of patient-specific anatomy to the interventional cardiologist.
| Impact on daily practice Structural disease demands a fully 3D appreciation of anatomy. In this regard, patient-specific 3D printed models can provide a useful adjunct in the care of patients with complex CHD. |
Funding
This work was supported by the National Institute for Health Research Cardiovascular Health Technology Cooperative and Biomedical Research Centre awarded to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. 3D echo showing the course of the fistula in case 4.
Moving image 2. Rotational angiography of the 3D model after closure device positioning.
Moving image 3. Post-release angiography showing AVP II in situ with no residual flow (case 4).
Supplementary data
To read the full content of this article, please download the PDF.
3D echo showing the course of the fistula in case 4.
Rotational angiography of the 3D model after closure device positioning.
Post-release angiography showing AVP II in situ with no residual flow (case 4).

