Herein we advocate for the controlled introduction of new coronary stents by means of cluster-randomised clinical trials as an integral part of the implementation process. The controlled introduction of new stents will expand the evidence base and mitigate concerns about the uncertain performance of stents that have been approved and implemented in clinical practice based on demonstration of non-inferiority rather than superiority compared to pre-existing stents1.
Contemporary coronary stent platforms are associated with good clinical outcomes, and it is generally considered sufficient for a new stent to perform “as well as” the best available stents for it to be introduced into clinical practice. New coronary stents are therefore evaluated in non-inferiority rather than superiority trials. For the new stent to be considered non-inferior (essentially “as good as”) and be approved by American and European regulatory bodies, it must be shown to not increase the risk of the primary endpoint by more than a prespecified non-inferiority margin compared to the previously approved stent (Figure 1). Put differently, the risk difference (or ratio) of the primary outcome with the new stent compared to the old stent must be significantly lower than a prespecified non-inferiority margin2. Unfortunately, the non-inferiority margins used in contemporary stent trials are higher than what many would consider a clinically meaningful risk difference, frequently extending well above a 50% relative risk increase compared to the control stent1. Therefore, a non-inferiority trial may be “positive” even if there is a substantial probability that the new stent carries a clinically significant greater risk of the primary outcome than the old stent1.
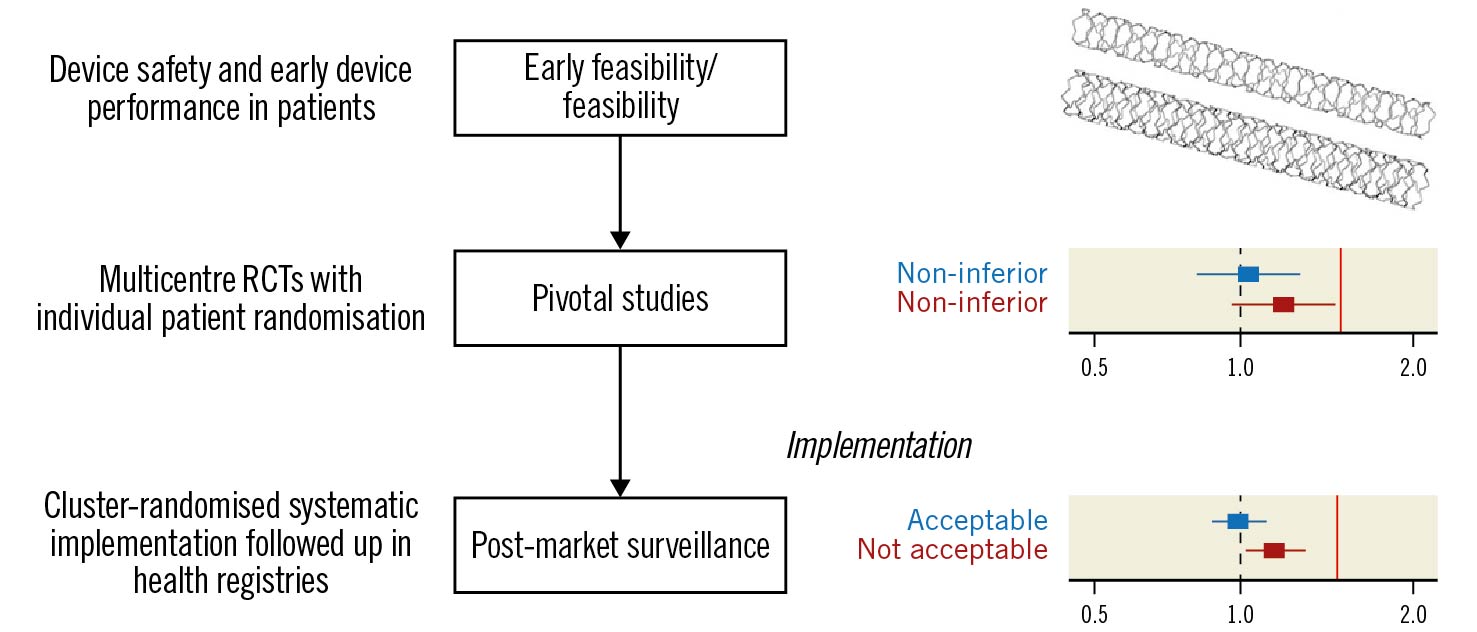
Figure 1. Cluster-randomised implementation strategies for post-market surveillance of coronary stents. By implementing new coronary stents in a systematic manner (e.g., by cluster randomisation) and following up outcomes in health registries, the evidence base at the time of implementation can be expanded with data from a very large number of patients at minimal cost. Whereas preapproval pivotal trials are rich in detail and provide an essential initial assessment of whether a new stent is comparable (non-inferior) to existing stents, post-approval cluster-randomised implementation strategies involving a large number of patients can achieve sufficient power to detect stents that are inferior, or superior, to currently used stents. Additional randomised data from a large number of patients can help confirm whether the performance of stents that were shown to be non-inferior in pivotal studies with limited sample size (middle panel, wide confidence intervals) is acceptable for continued use in large real-world cohorts (bottom panel, narrower confidence interval). The red vertical lines represent the non-inferiority margin (i.e., the criteria for meeting non-inferiority). RCTs: randomised controlled trials
Whereas “accepting” a relative risk as high as 50% as “non-inferior” may seem odd, the trial investigators and regulatory bodies must balance using a too permissive margin (resulting in uncertainty as to whether the new stent really can be considered “as good as”) versus using a too strict non-inferiority margin (resulting in an impossibly large number of trial participants and costs to afford sufficient statistical power).
Even with a permissible non-inferiority margin, the demonstration that the relative risk of adverse events with the new versus the old stent is unlikely to be greater than the non-inferiority margin is somewhat reassuring; but it should not be considered sufficient for widespread implementation of that stent platform without further evaluation. Furthermore, the 95% confidence interval of the risk difference (i.e., the uncertainty regarding the effect of the new versus the old stent) frequently includes the possibility not only of a clinically significant excess risk with the new stent, but also the possibility of a clinically relevant risk reduction. It is therefore warranted to acquire greater certainty regarding how a new stent performs relative to older stents after the stent is approved and implemented in clinical practice, to ensure that patients can be offered the best and most cost-effective stents.
Fortunately, the increasing number of health care registries that track outcome data in contemporary cardiology present a cost-effective opportunity to collect outcome data3. These health care registries can be used as data capture systems to allow a robust but inexpensive evaluation of new stents if the implementation of these stents is done systematically using a cluster-randomised approach.
Different types of cluster-randomised designs may be chosen for different settings. Individual hospitals or health care regions (clusters) may change from the comparator stent to the new stent in a randomly assigned order (stepped wedge cluster design); be assigned to either the comparator stent or the new stent (parallel group cluster design); or be randomised to treatment with one of the stents for the first half of the study period and the other stent for the remainder of the study (crossover cluster design)4.
By systematically introducing new stents using a cluster-randomised approach and following up outcomes in already existing health registries, we can acquire meaningful data for a substantial number of patients at minimal cost. As an example, more than 25,000 stents are typically implanted in over 15,000 patients every year in Sweden alone. Cluster-randomised implementation of new stents thus represents an effective means of post-market surveillance, an important part of the evidence-generating process for contemporary coronary stents that is required by European regulations (Medical Device Regulation [MDR] 2017/745), but that has been relatively neglected5.
To reliably evaluate the implementation of a new treatment, several criteria must be met. First, it must be possible to define the desired study population using the intended registries. In other words, patients for whom the treatment is not indicated should not be included in the study population. Second, treatment assignment must be unrelated to the likelihood that a patient is captured in the registries and classified as belonging to the desired patient population; and third, the key outcome measures must be reliably obtained from the registries, and outcomes must not be more likely to be reported in the registry in either treatment arm. Some nationwide health care registries, such as the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), fully fulfil these criteria whereas others do not3.
An example of a cluster-randomised design to evaluate the implementation of a new cardiovascular treatment in clinical practice is SWITCH-SWEDEHEART (ClinicalTrials.gov: NCT05183178), in which the transition from ticagrelor to prasugrel as the preferred P2Y12 antagonist for treatment of patients with acute coronary syndrome is systematically evaluated. Several Swedish health care regions represent clusters that were randomly assigned to change from ticagrelor to prasugrel in sequential order, with one cluster changing from ticagrelor to prasugrel every 9 months. Variables to define the study population as well as baseline characteristics and the primary and secondary clinical outcomes will be acquired from the Swedish health care registries. Except for time commitment and minor costs related to ensuring timely implementation of the change from ticagrelor to prasugrel, the study is estimated to enrol 12,000 patients at minimal cost.
Similar cluster-randomised evaluations of the implementation of new stents represent inexpensive means of expanding the evidence base for these stents. Arguably, cluster-randomised implementation of a new stent is logistically much easier to perform than the SWITCH-SWEDEHEART study, since stents are implanted at a limited number of percutaneous coronary intervention centres by a limited number of interventional cardiologists.
Lastly, it is important to emphasise that although we believe that pragmatic registry-based cluster randomisation presents a unique opportunity to evaluate the implementation of new stents in clinical practice once these stents are approved, traditional individual patient randomisation is the study design that is most robust to bias and should remain the basis for approval of new stents. Compared to individual randomisation, cluster randomisation has less precision and may increase the risk of type II errors, and follow-up of events through registries is more likely to overlook or misclassify events than rigorous trial-specific adjudication processes. Most importantly, for cluster randomisation to be effective and reliable, the requirement for individual informed consent essentially needs to be waived; and such cluster-randomised comparisons of stents should therefore generally only be undertaken when both stents are fully approved for clinical use with indications for the intended study population, and after careful consideration of any undue risks4.
In summary, cluster-randomised implementation of new stents after they are approved represents a complementary process to preapproval individual patient randomised controlled trials that can substantially expand the evidence base and safeguard against the long-term use of inefficacious or unsafe stents.
Conflict of interest statement
O. Angerås reports grants and personal fees from Abbott Vascular, and personal fees from Boston Scientific, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

