
In this issue of EuroIntervention, Biswas et al examine the characteristics of the many regional and national percutaneous coronary intervention (PCI) registries and discuss those registries’ choices of measured outcomes1.
The authors note that nearly 75% of all PCI registries were established after 2000. Although many collect data on PCI and diagnostic angiography procedures, a large minority collect data on PCI procedures only. There is also widespread variation in collection of metrics and outcomes: some registries collect only in-hospital outcomes, while others collect long-term post-PCI outcomes. Few are linked to administrative data or adjust outcome reporting based on patient risk; roughly 40% provide publicly available data, with some opting not to make data identifiable to an individual operator or hospital. We congratulate the authors on a thorough review of the current state of PCI registries that raises many provocative questions.
Beginning with the Society of Thoracic Surgeons (STS) national database in 1989, cardiovascular medicine has set the standard for medical registries. First initiated in response to pressure from payers and state governments demanding evidence of quality for expensive procedures, cardiovascular registries now serve multiple purposes, including benchmarking physician and institutional quality, measuring patient outcomes, assessing adherence to guidelines and performance measures, and conducting registry-based randomised clinical trials (RCTs). However, as cardiovascular medicine moves into the digital era, we must consider how the cardiovascular community can optimise the value of registries.
The fundamental justification for registries has been the belief that implementing evidence-based guidelines and learning from experience could improve patient outcomes, allow quality to be assessed, and provide credible evidence for the value of procedures and medications. When guidelines have been based on high-quality evidence2,3, this approach has proven effective. However, when guidelines are based on poor-quality evidence - as happened with high-dose erythropoietin4 - harm can ensue. Now, as registries are integrated into an increasingly data-rich environment, they are best viewed within a broader evidence framework for learning and quality.
Registries are spreading throughout medicine and healthcare; in particular, astute patient advocacy groups and clinician organisations are developing registries not just for quality improvement but to understand the natural history of diseases and identify volunteers for clinical trials. While many opportunities exist for such multiple-use registries, the lack of standardised metrics across registries and the laborious data collection required have limited their usefulness, including many of the PCI registries reviewed by Biswas et al1. Although reliable data to inform quality, guide care, and identify knowledge gaps would be beneficial in any disease context, no health system can afford the time and resources needed for data collection if each registry exists in isolation.
Although many PCI registries are voluntary, some are required to maintain catheterisation laboratory accreditation5. With the rise of public reporting of PCI performance and outcome metrics, there is mounting evidence that clinicians feel pressured to pick cases with a high probability of good outcome rather than an optimal treatment benefit. In this scheme, the sickest patients may not get appropriate treatment because mortality rates will be high even if the overall treatment effect is highly beneficial6-8. If performance data are not appropriately adjusted for patient risk and consciously inclusive of the impact on overall outcomes, public reporting could encourage risk-averse behaviour by PCI operators. As the present study shows, less than one third of PCI registries provide risk-adjusted mortality rates, while 20% publicly report outcomes identifiable to an individual hospital, leading to concern that institutions caring for higher-risk patients may be inappropriately labelled as poor performers. A recent analysis of revascularisation and in-hospital mortality rates among U.S. states with post-procedure public reporting compared with states without such reporting showed that patients in states with public reporting were less likely to undergo revascularisation and had higher in-hospital mortality6. Further, an examination of changes in revascularisation rates after New York State began excluding public reporting of PCI outcomes in patients with cardiogenic shock demonstrated a significant increase in the use of PCI for cardiogenic shock and a decrease in in-hospital mortality rates8. It is therefore crucial for registries to scrutinise the impact of reporting post-procedural outcomes on care practices and patient selection in their respective country or region. Further work is needed to determine the most appropriate means for adjusting risk, as there is concern that risk-adjusted mortality rates are simplistic and may not perform well in high-risk contexts9,10.
The future of PCI registries
As new registries proliferate, methods to derive registry data from electronic health records (EHRs) and administrative claims will be needed. According to one estimate, registry participation for some health systems can require an investment of >US $100,000 and necessitate considerable data input from medical staff11. However, automating this process is not trivial, and the medical terminology used in most EHRs does not easily harmonise with registry data collection tools. Many institutions are now developing protocols for data input to implement a sustainable model of data collection12.
Embedding RCTs into registries can reduce effort and cost. SAFE-PCI for Women, an early example of an RCT embedded into an existing registry13, was embedded into the National Cardiovascular Data Registry (NCDR) CathPCI Registry, thus reducing site coordinator workload by roughly 65%14. Registry-based RCTs also promise to enrol more “real-world patients” (given less stringent eligibility criteria) and allow investigators to perform patient monitoring and follow-up in real time. Registry-based RCTs such as the TASTE trial have benefited from leveraging a national registry to examine in real time what proportion of patients was being enrolled in the trial15.
With >20 million patients already entered into PCI registries and many scheduled to be entered each year1, there is an opportunity to overcome cultural boundaries and create a learning system for PCI unlimited by national or regional boundaries. Continuous learning about the most successful approaches to PCI, and incorporation of randomisation to inform important decisions about diagnostic and treatment options, could generate knowledge and improve practice at a substantially accelerated pace.
A system in which clinical records generate a registry with every procedure, combined with follow-up gleaned directly from digital records and patient self-reports, offers a method of conducting multiple registries for different aspects of cardiovascular disease, conditions and procedures that should be characterised by registries. A common core of information (demographics; medication lists; problem and procedure lists) can be shared across many diseases. Although medication dispensing information is readily available, device identifiers are only now becoming commonplace and need to be adopted rapidly. Proof-of-concept exists with the initial phase of the National Patient-Centered Clinical Research Network (PCORnet), where data from >100 million Americans are curated quarterly16.
Biswas and colleagues illustrate the widespread variation in practices and reporting among PCI registries worldwide. Although these registries are powerful tools for examining patient care and procedural outcomes, changes are needed to make these tools feasible and useful for health systems (Figure 1). An examination of what outcomes are appropriate for public reporting and how risk adjustment should be addressed is important to avoid adverse changes in health system and operator behaviour that ultimately impact patient care. Now is the time to begin serious migration of registries to data collection directly from the EHR and claims so that clinicians and institutions are not overwhelmed by clerical burdens. Overcoming national and regional cultural barriers to data sharing offers the promise of very rapid learning in PCI procedures, using a combination of constant observation for trends combined with registry-based RCTs to define which treatments are best for which patients.
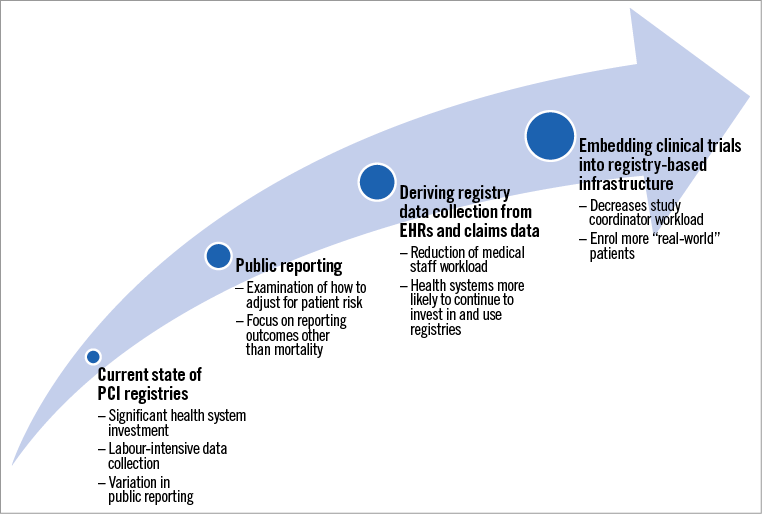
Figure 1. The current state of PCI registries and components of evolution.
Conflict of interest statement
R. Califf was the Commissioner of Food and Drugs for the US Food and Drug Administration from February 2016 to January 2017 and Deputy Commissioner for Medical Products and Tobacco for the US Food and Drug Administration from February 2015 to January 2016. R. Califf serves on the corporate board for Cytokinetics. He reports receiving consulting fees from Merck, Amgen, Biogen, Genentech, Eli Lilly and Boehringer Ingelheim and is employed as a scientific advisor by Verily Life Sciences (Alphabet). He is Chair of the Board of the People Centered Research Foundation. J. Rymer reports receiving a career development award from the American College of Cardiology and grant support from Abbott Pharmaceuticals.

