
Abstract
Aims: Clinical registries have a growing role in the assessment of healthcare quality and safety. It is unclear, however, how many countries utilise registries for patients who receive percutaneous coronary intervention (PCI). The aim of this review was to provide an overview of the characteristics of PCI registries from around the world.
Methods and results: A systematic search of the published and online grey literature was undertaken to identify currently active national PCI registries. In countries without a national PCI registry, the three largest regional registries were included. Thirty registries in 26 countries that met inclusion criteria were identified, of which 24 (80%) are national registries and six (20%) are regional registries. Fourteen registries (47%) collect 30-day mortality rates while 11 registries (37%) collect 12-month mortality rates. Nine registries (30%) provide risk-adjusted mortality rates and 16 registries (53%) report bleeding outcomes, utilising a variety of bleeding definitions. Thirteen registries (43%) publicly report key quality metrics.
Conclusions: There is substantial geographic variation in the distribution of PCI registries. Comparison across registries is challenging due to varying data definitions and collection time points. Public reporting of outcomes data is being increasingly implemented by PCI registries, but risk-adjustment models remain underutilised.
Abbreviations
NCDR: National Cardiovascular Data Registry
PCI: percutaneous coronary intervention
RCT: randomised controlled trial
Introduction
Worldwide, there has been an increasing emphasis by healthcare regulators on measuring and improving the quality of medical care. While results from randomised controlled trials (RCTs) provide the highest level of evidence regarding the efficacy of interventions, they have well recognised limitations. RCTs may not always reflect “real-world” medical settings and often underrepresent significant portions of the community, such as women and the elderly1. Clinical registries have consequently emerged as a powerful tool to assess healthcare effectiveness and safety and improve quality of care, as well as to inform on the real-world impact of new interventions or medications outside the confines of RCTs2. Over the last two decades, there has been a substantial growth in national and major regional percutaneous coronary intervention (PCI) registries, predominantly in developed countries. However, many countries, particularly low- and middle-income countries, have been slow to adopt large-scale multicentre clinical registries, potentially due to concerns about costs and a lack of a clearly defined utility and benefit3. Health regulators and funding agencies have also placed greater emphasis on public reporting of hospital and/or operator outcomes, particularly in the area of PCI, to assess performance and clinical quality4. This has led to several concerns including misinterpretation of data by healthcare users, leading to avoidance of so-called low-performing hospitals5. In addition, it may potentially lead to provider “risk-averse behaviour” whereby PCI may not be offered to the most high-risk patients who may paradoxically have the most to gain from timely treatment.
In the current environment of demand for big data and an evolving role for registries, it is appropriate to examine the current status of PCI registries. This review, therefore, aims to provide an overview of the distribution and characteristics of active PCI registries from around the world, and to describe the associated PCI registry concepts including their approach to clinical outcome measurement, risk adjustment and public reporting.
Methods
A structured literature review was performed by searching the PubMed database in January 2018 using the keywords “percutaneous coronary intervention” and “registry” (Figure 1). Our search was restricted to manuscripts published in English.
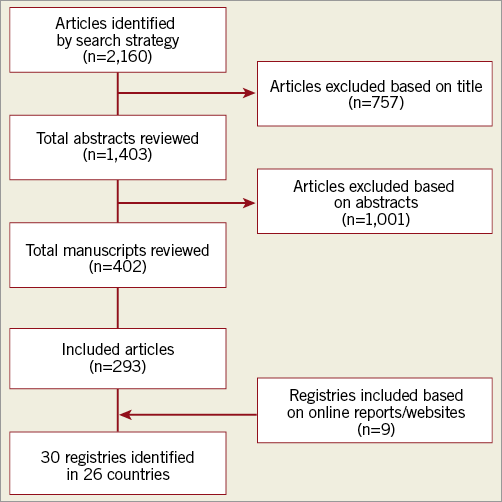
Figure 1. Flow diagram illustrating literature search strategy.
For the purposes of article selection, a PCI registry was defined as a dedicated multicentre database systematically collecting information on clinical and procedural details of patients undergoing PCI2. Only registries currently actively collecting data at either a major regional or national level, for all-comers undergoing PCI were included. We considered a registry to be “national” if it was reported as the accepted countrywide system for data collection on PCI and had published reports or publications. In countries without a national PCI registry, up to three of the largest regional registries were included. One author (S. Biswas) reviewed the titles and abstracts of all articles to identify suitable registries. If there were any uncertainties regarding whether an article or registry met inclusion criteria, a full article review was conducted. All selected articles and included registries were then subsequently verified by a second author (D. Stub).
An additional internet search of webpages was conducted in January 2018, using the Google Advanced Search facility with the term “percutaneous coronary intervention registry”. Also, the names of all United Nations member countries with the term “percutaneous coronary intervention registry” were searched for in Google. In countries where no national PCI registry was identified, a second search was performed with the name of the capital city and the term “percutaneous coronary intervention registry”, to identify any major regional registries. Any registries identified using this strategy that met the inclusion criteria were included. Additional information on identified registries was also obtained by using the name of the registry as the search term. Two authors (S. Biswas, D. Stub) then reviewed the results to ensure that no eligible registries were missed. As not all registries had published protocol papers or websites available in English, the information on their data sets may not be representative of the complete set of variables collected. Further information about search criteria can be found in Supplementary Appendix 1.
For each registry that was identified as meeting the inclusion criteria, data were collected on whether registry participation was voluntary or mandatory, the time points at which mortality data were collected, as well as the definition of bleeding used. Furthermore, whether the registries provided public reporting of outcomes at either a hospital or operator level (both identified and deidentified) was also ascertained. All identified registries were also contacted by email to complete a pre-specified questionnaire to confirm our data, out of which 10 registries (33%) provided a response.
This review has been registered with PROSPERO (registration no. CRD42018090574).
Results
The literature search identified 30 PCI registries that met the inclusion criteria located in 26 countries: 24 (80%) are national registries and six (20%) are regional registries (Figure 2)6-21. In Australia and Canada, there are no national PCI registries; therefore, only major regional registries in these countries were included22-27. While the earliest PCI registry was established in 1990, 22 out of the 30 registries (73%) were established in or after 2000 (Figure 3). In total, our conservative estimate indicates that over 20 million patients undergoing PCI have been included in PCI registries across the world to date. Overall, 12 registries (40%) collect data on PCI procedures only, while 18 registries (60%) also collect data on patients undergoing diagnostic coronary angiography without PCI (Table 1). Estimated case coverage is variable but is generally more complete where participation is mandated by government compared to when participation is voluntary. Fifteen registries (50%) are associated with a government organisation, while the other 15 registries (50%) are associated with a national society of cardiology (Supplementary Table 1). All but three registries (the National Interventional Council Registry of India, the Austrian National Cathlab Registry and the Swiss Working Group of Interventional Cardiology PCI survey) prospectively collect individual patient-level data13,28,29.
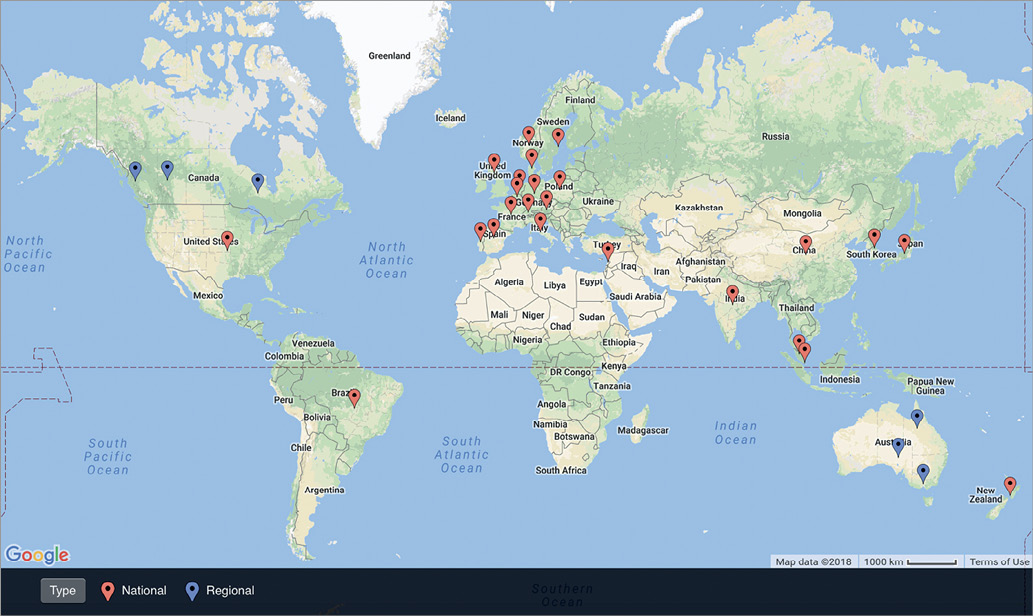
Figure 2. Map of national- and regional-level percutaneous coronary intervention registries.
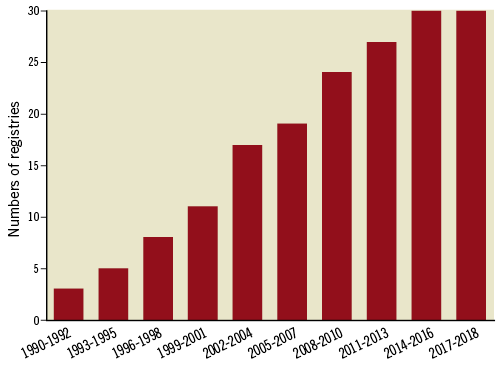
Figure 3. Growth of percutaneous coronary intervention registries over time.
Twenty-nine registries (97%) collect in-hospital mortality rates which range from 0.5% to 2.5% for all PCI (Figure 4) and from 2.5% to 6.9% for PCI in ST-elevation myocardial infarction (Supplementary Figure 1). Fourteen registries (47%) collect mortality rates at 30 days, while 11 registries (37%) collect mortality rates at 12 months following the index PCI (Table 1). The majority of registries utilise individual record review for all variables, including mortality data at follow-up (Supplementary Table 2). Eleven registries (37%) obtain mortality data through linkage with national administrative or mortality databases30-33. Nine registries (30%) provide risk-adjusted mortality rates, although the covariates used in risk models vary22,23,34,35. Sixteen registries (53%) report bleeding outcomes, with a variety of bleeding definitions utilised (Supplementary Table 1)14,23,36.
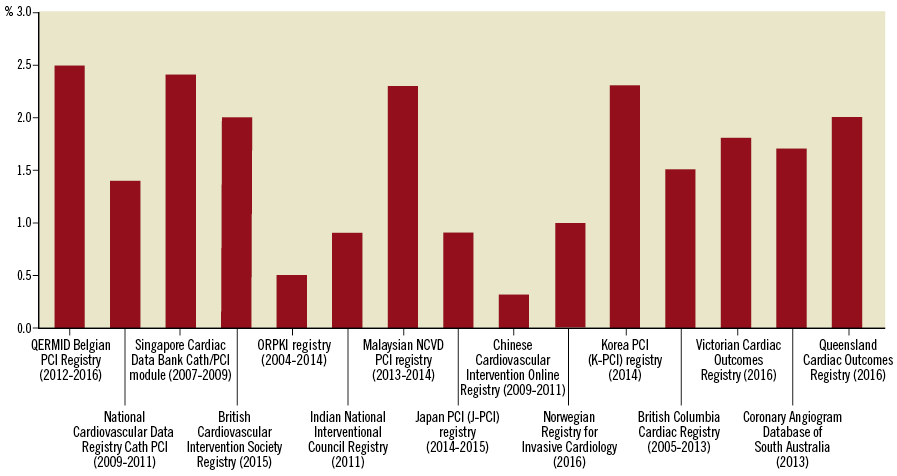
Figure 4. In-hospital mortality rate after percutaneous coronary intervention across the registries.
Thirteen registries (43%) provide publicly available reports of their data at hospital or operator level. Two of these registries (Victorian Cardiac Outcomes Registry and Spanish Cardiac Catheterization and Coronary Intervention Registry) anonymise all data such that no hospital can be individually identified (Supplementary Figure 2)6,27. Six registries (20%) publicly report mortality data which are identifiable to an individual hospital, while the British Cardiovascular Intervention Society registry publicly reports mortality data that are identifiable to an individual operator. Four registries (13%) publicly report quality measures other than mortality that are identifiable to a hospital such as case mix, door-to-balloon time and prescription of guideline-directed secondary prevention therapy15,29,30,37,38.
Discussion
Over the last two decades, there has been a substantial increase in the number of PCI registries. While nearly all PCI registries collect in-hospital mortality data, a much smaller proportion collect 30-day and 12-month mortality data. Bleeding complications are only reported by just over half of all PCI registries; a variety of bleeding definitions is used. Public reporting of key quality metrics and outcome data is being increasingly implemented but risk-adjustment models appear to be underutilised by PCI registries.
ROLE AND UTILITY OF CLINICAL REGISTRIES
The growth of cardiac registries over the last two decades has been in parallel with the steady development of clinical quality metrics in cardiovascular diseases since the early 1990s when a national effort to measure the quality of care for American patients with acute myocardial infarction was initiated39. Clinical registries are able to collect comprehensive data systematically on large numbers of patients in real-world practice, and therefore may be used to measure achievement of quality standards and adherence to guidelines40.
However, the impact of clinical registries on hard clinical outcomes, such as survival, has been mixed. The establishment of lung and colon cancer registries in Denmark and Manchester, England, respectively, was found to be associated with improved survival of patients with those conditions, probably due to better quality of care after the introduction of the registries41,42. Similarly, a reduction in trauma-related mortality was noted following introduction of systematic data collection and monitoring in the Victorian Statewide Trauma Registry in Australia43. However, a registry established to monitor acute stroke care in Germany did not demonstrate any improvement in mortality from stroke over time44. On the other hand, the impact of registries on improving systems of care or adherence to guidelines has been largely positive. Participation in heart failure registries in America has been shown to be associated with increased use of evidence-based heart failure therapies, shorter length of stay for patients hospitalised with heart failure and reduced in-hospital morbidity and mortality at both patient and hospital level45,46.
DATA STANDARDISATION
With the rapid growth in PCI registries operating across the world, the opportunity to compare outcomes of patients treated with PCI in different countries has been of particular interest20. As a result, some newer PCI registries, such as the Coronary Angiogram Database of South Australia registry, have been designed based on other large registries to facilitate this comparison and international benchmarking25. However, our review highlights that there is large variation in the outcomes measured and definitions of outcomes across the registries worldwide, which limits international comparisons2.
To address this issue, a number of expert committees have been formed to develop standard definitions and outcome measures47,48. The overall consensus has been that survival should be assessed at 30 days post discharge, as well as annually up to five years after the index event. They also recommended collection of patient-reported outcome measures which are currently performed by only a very few registries. Despite this, our analysis indicates that only about a third of all registries report outcomes beyond 30 days post PCI.
PUBLIC REPORTING AND RISK ADJUSTMENT
It has been proposed that public reporting of procedural outcomes will provide more transparency and accountability of healthcare providers, as well as provide poorly performing hospitals or operators with an incentive to improve their performance49. Following the introduction of public reporting of risk-adjusted mortality after coronary artery bypass graft surgery in the late 1980s in New York, a study comparing 30-day mortality between 1994 and 1999 among New York and non-reporting states showed that patients in non-reporting states were 52% more likely to experience short-term mortality, after adjusting for preoperative illness severity50. However, while most studies have shown a positive association between public reporting and improvement in key quality metrics, several studies have reported that the use of PCI in patients with myocardial infarction was lower in states with public reporting compared with non-reporting states, especially in the highest risk patients such as those with cardiogenic shock and post-cardiac arrest51,52. Surveys performed on interventional cardiologists have also confirmed that the knowledge that their PCI mortality rates will be made public affects their decision to perform PCI53. Therefore, public reporting has the potential to become counterproductive in improving outcomes after PCI as it may be increasing operator risk-averse behaviour and withholding of PCI from the highest risk patients who also potentially stand to gain the most benefit from it54.
One suggested strategy to minimise the potential adverse impact of public reporting of outcomes has been to report risk-adjusted mortality rates only, to account for high-risk patients in whom outcomes after PCI are often poor due to high preprocedural risk5. However, the present review found that less than one third of all PCI registries report risk-adjusted mortality rates. There is also significant variability in the nature and complexity of the risk-adjustment models used by the different registries55. In an analysis of six different risk models used in patients undergoing high-risk PCI with haemodynamic support, all models were found to have poor predictive ability for mortality55. While adding in variables such as frailty may improve model validity, it is important to accept that no risk-adjustment model will be perfect5. Therefore, changing the focus from risk-adjusted mortality rates to reporting more process-oriented measures such as guideline-recommended medication prescription on discharge, as is done currently by the NCDR Cath/PCI registry, should be considered in the future.
Limitations
The present review has a number of limitations. First, all searches were performed in English. While a broad search strategy was used to minimise publication bias, it is possible that some registries may have been missed. In addition, many registries only published online reports in their native language, thereby potentially affecting interpretation of their key characteristics. To mitigate this risk, all individual registries were contacted to confirm the findings on their key characteristics. Finally, based on the pre-specified focus of the review to include national or the three largest regional registries only, a number of high-quality regional registries were not included in the analysis.
Conclusions
Our review demonstrates that the global distribution of PCI registries is patchy, with the highest concentration of registries in Europe. Clinical PCI registries have a key role to play in improving the quality of local cardiovascular care, but comparison across regions and countries may be challenging due to varying registry definitions and data collection time points. While public reporting of key quality metrics may help to improve processes and outcomes, registries must consider how to avoid risk-averse behaviour with appropriate and sophisticated risk adjustment.
| Impact on daily practice PCI registries have a key role to play in improving the quality of cardiovascular care, but there is significant geographic variation in their use. Standardisation of data definitions may help to enhance their role in the future, particularly for international comparisons. Public reporting of key quality metrics may help to improve outcomes, but registries must be cautious that public reporting does not encourage operator risk-averse behaviour by avoiding treatment of high-risk patients who stand to gain the most benefit from intervention. |
Funding
No specific funding was received for the work in this manuscript. However, we acknowledge the following sources of scholarship/grant support: The National Heart Foundation of Australia (S. Biswas: reference number 101518; D. Stub: reference number 101908), the Australian Government Research Training Program (S. Biswas), the National Health and Medical Research Council (C.M. Reid: reference no. 1045862), and the Viertel Foundation Clinical Investigator award (D. Stub).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Figure 1. Approach to public reporting of outcomes by different registries.
Supplementary Figure 2. In-hospital mortality post PCI for STEMI across the registries.
Supplementary Table 1. Organisational data for included registries.
Supplementary Table 2. Outcomes data collection in included registries.
To read the full content of this article, please download the PDF.

