Abstract
Aims: In this study we sought to evaluate the clinical impact of the residual SYNTAX score (rSS) after percutaneous coronary intervention (PCI) in patients with chronic total occlusion (CTO) and multivessel coronary artery disease (CAD).
Methods and results: We analysed data from 1,043 patients with CTO and multivessel CAD who were treated with PCI or coronary artery bypass grafting (CABG). Patients were divided into three groups: patients with rSS≤12 after PCI (rSS≤12 group, n=445), patients with rSS>12 after PCI (rSS>12 group, n=150), and patients who underwent CABG (CABG group, n=448). We compared the incidence of cardiac death among the three groups. During a median follow-up period of 42 months, cardiac death occurred in 14 patients (3.1%) in the rSS≤12 group, 14 patients (9.3%) in the rSS>12 group, and 29 patients (6.5%) in the CABG group. On multivariate analysis, the rSS≤12 group had a significantly lower incidence of cardiac death than the rSS>12 group (hazard ratio [HR] 0.35, 95% confidence interval [CI]: 0.16 to 0.75; p=0.01), but had an incidence of cardiac death similar to that of the CABG group (HR 0.63, 95% CI: 0.32 to 1.23; p=0.17).
Conclusions: An rSS≤12 after PCI may reduce the risk of cardiac mortality and could be a measure of reasonable incomplete revascularisation in patients with CTO and multivessel CAD.
Abbreviations
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CTO: chronic total occlusion
MI: myocardial infarction
PCI: percutaneous coronary intervention
rSS: residual SYNTAX score
SYNTAX: Synergy between PCI with Taxus and Cardiac Surgery
Introduction
Several studies have reported the long-term survival benefit of coronary revascularisation in patients with chronic total occlusion (CTO)1,2. Although complete revascularisation is desirable in patients with coronary artery disease (CAD), complete revascularisation is not always possible due to the complexity of CTO lesions and the potentially lethal complications associated with revascularisation procedures. Recent studies have proposed the concept of reasonable incomplete revascularisation after percutaneous coronary intervention (PCI) in stable CAD, and incomplete revascularisation could be considered a rational treatment strategy3,4. The residual SYNTAX score (rSS) was designed and validated to quantify the absolute amount of untreated CAD after PCI5-7, and an rSS>8 was identified as a level of incomplete revascularisation strongly associated with increased mortality and adverse ischaemic events in patients with stable multivessel CAD5. However, few studies have examined the clinical impact of rSS after PCI in patients with CTO and identified a reasonable degree of incomplete revascularisation using the rSS. In this study, we evaluate the impact of rSS after PCI and quantify risk reduction in patients with CTO and multivessel CAD.
Methods
STUDY POPULATION
Between March 2003 and February 2012, 2,024 consecutive patients were enrolled in the Samsung Medical Center CTO registry. The inclusion criteria for the registry were: 1) at least one CTO lesion detected on diagnostic coronary angiography, and 2) symptomatic angina and/or a positive functional ischaemia test. Exclusion criteria were: 1) previous coronary artery bypass grafting (CABG), 2) a history of cardiogenic shock or cardiopulmonary resuscitation, and 3) ST-segment elevation acute myocardial infarction (MI) during the preceding 48 hours1.
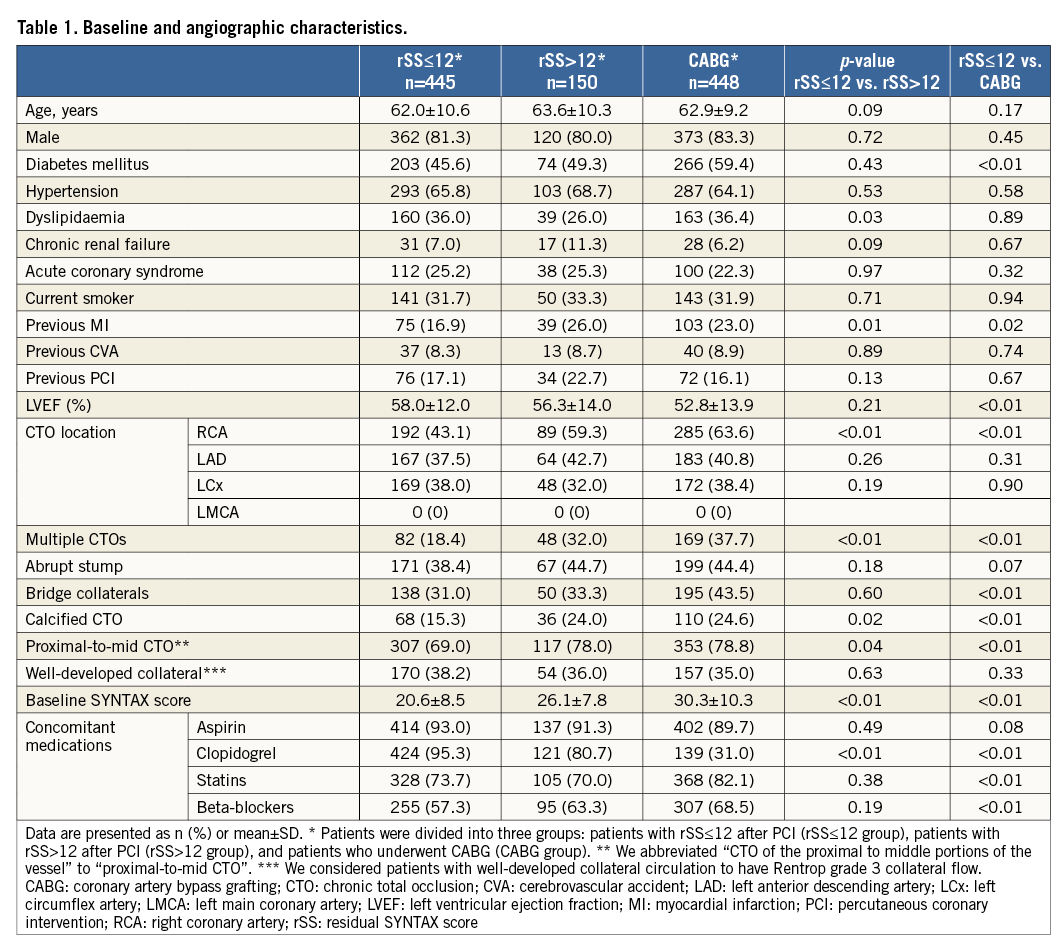
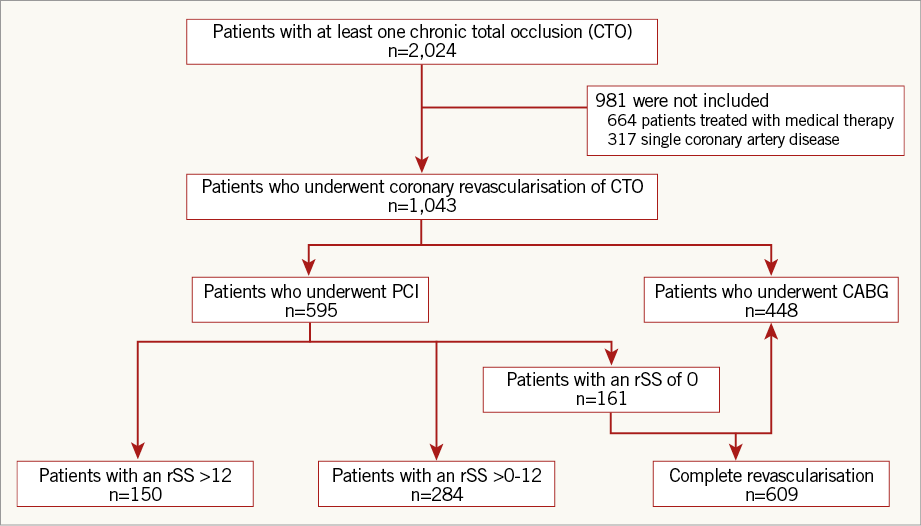
Of the patients included in the registry, 1,043 patients who underwent coronary revascularisation of CTO were included in the final analysis, while patients with single-vessel CAD or medical therapy alone were excluded. We evaluated using a reasonable value of 12 score (12 score=8 score [rSS cut-off value in the original SYNTAX trial]+4 score [mean additional SYNTAX score arose from CTO lesion in our registry]) and patients treated with CTO-PCI were divided into two groups, patients with rSS≤12 after PCI (rSS≤12 group) and patients with rSS>12 (rSS>12 group) (Figure 1). We compared the incidence of clinical outcomes between the two groups and patients who underwent CABG (CABG group). The Institutional Review Board of the Samsung Medical Center approved this study and waived the requirement for informed consent.
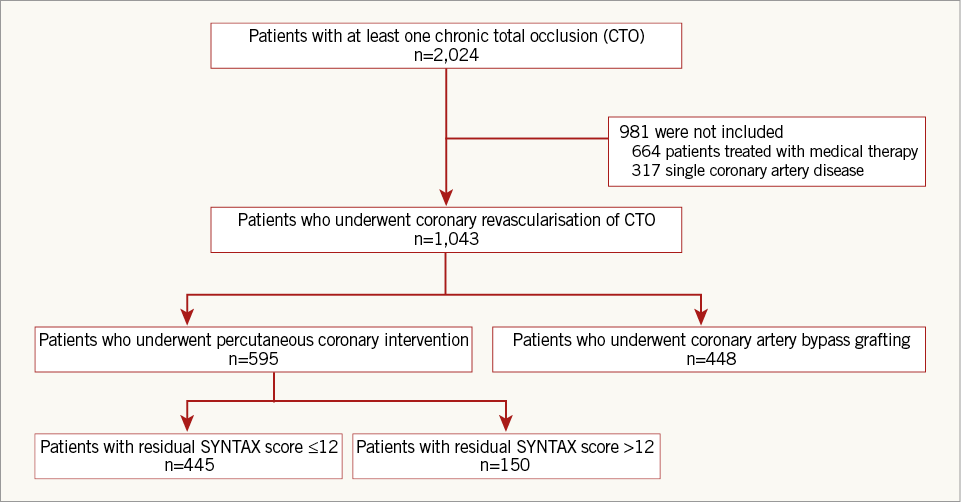
Figure 1. Schematic of group distribution. CABG: coronary artery bypass grafting; CTO: chronic total occlusion
TREATMENT STRATEGY
All patients received antiplatelet therapy with 81 to 325 mg aspirin or 75 mg clopidogrel daily. Revascularisation of CTO was accomplished by CABG or PCI with a drug-eluting stent (DES), and the final revascularisation strategy was selected based on patient and physician preferences. Arterial grafting with an off-pump coronary artery bypass was the preferred technique for CABG. PCI was performed using contemporary techniques such as bilateral injections, a specialised stiff, hydrophilic wire with a tapered tip, microcatheters, and a retrograde approach when available. The decision to pursue invasive treatment, access-site selection and DES type, use of intravascular ultrasound, and use of glycoprotein IIb/IIIa receptor inhibitors were left to the operator’s discretion. All interventions and procedural anticoagulation were performed according to current standard guidelines. All patients received loading doses of aspirin (300 mg) and clopidogrel (300-600 mg) before PCI unless they had previously received these antiplatelet medications. Aspirin treatment was continued indefinitely, while the duration of clopidogrel treatment was left to the discretion of the individual physician. Successful PCI was defined as a final residual stenosis less than 20% of the vessel diameter with TIMI flow grade ≥2 after revascularisation, as assessed by visual estimation. CABG was considered successful when all vessels with significant lesions, including CTO, were bypassed16,17.
STUDY OUTCOME AND DEFINITIONS
The primary outcome was cardiac death during follow-up. Secondary outcomes were all-cause death, MI, cerebrovascular accident (CVA), repeat revascularisation and major adverse cardiac and cerebrovascular events (MACCE). Clinical events were defined based on recommendations from the Academic Research Consortium10. All deaths were considered to be of cardiac origin unless a definite non-cardiac cause could be established. MI was defined as an increase in creatine kinase MB fraction or a value of troponin T/troponin I greater than the upper limit of normal, with concomitant ischaemic symptoms or electrocardiography findings indicative of ischaemia. Periprocedural enzyme increase was not included in this definition of MI. Repeat revascularisation was a composite of target lesion revascularisation (TLR), target vessel revascularisation (TVR), and non-TVR treated with PCI or CABG. MACCE was defined as a composite of all-cause death, MI, CVA, and repeat revascularisation. TLR was repeat PCI of the lesion within 5 mm of the deployed stent or bypass graft surgery for the target lesion. TVR indicates repeat revascularisation of the target vessel by PCI or CABG, whereas non-TVR indicates repeat revascularisation of any vessel other than the target vessel by PCI or CABG. Multivessel disease was defined as coronary lesions with ≥50% diameter stenosis in at least two of the three major epicardial coronary arteries or their major branches greater than 2.0 mm in diameter.
BASELINE, RESIDUAL, AND DELTA SYNTAX SCORES
Calculation of baseline SYNTAX scores11,12 was performed by personnel in the angiographic core laboratory (Heart Vascular and Stroke Institute, Samsung Medical Center, Seoul, Republic of Korea), with investigators blinded to treatment assignment. Baseline and procedural coronary angiograms were centrally stored and were analysed side-by-side by a panel of three interventional cardiologists blinded to clinical outcomes. Baseline SYNTAX score and its components, including anatomic location of all lesions recorded by the core laboratory in the calculation of the original SYNTAX score, were used to identify all coronary lesions in the baseline and procedural coronary angiograms. rSS was calculated based on the remaining obstructive coronary disease after PCI7. Intraobserver variability for calculation of rSS values (quartile partitioning) was based on re-analysing 50 cases at three-month intervals, and indicated a high level of observer agreement. Delta SYNTAX scores, representative of the burden of disease removed by PCI, were calculated by subtracting the rSS from the baseline SYNTAX score. The high level of agreement can be attributed to the fact that all members of the panel had knowledge of coronary lesion locations prior to PCI.
DATA COLLECTION AND STATISTICAL ANALYSIS
Clinical, angiographic, procedural and outcome data were collected using a web-based reporting system. Additional information was obtained by reviewing medical records or by telephone contact, if necessary. All baseline and angiographic data were reviewed and quantitatively analysed at the core laboratory with an automated edge detection system (Centricity CA 1000; GE Healthcare, Waukesha, WI, USA) using standard definitions13. All statistical analyses were performed using the intent-to-treat principle. Continuous variables were compared using the Student’s t-test or the Wilcoxon rank-sum test. Results were presented as mean±standard deviation (SD), or median with interquartile range. Categorical data were tested using Fisher’s exact test or the chi-square test. Cumulative event rates were estimated using the Kaplan-Meier method, and treatment effects were assessed using stratified log-rank statistics. Covariates that were either statistically significant upon univariate analysis or clinically relevant were included in multivariate models. All tests were two-tailed, and a p-value <0.05 was considered statistically significant. All analyses were performed with the Statistical Analysis Software package, Version 9.2 (SAS Institute, Cary, NC, USA).
Results
BASELINE AND ANGIOGRAPHIC CHARACTERISTICS
Of 1,043 patients with at least one CTO lesion and multivessel CAD, 595 patients were treated with PCI (57% of the study population), and 448 patients were treated with CABG (43.0%). Mean baseline SYNTAX scores were 22.0±8.6 and 30.3±10.3 in patients who underwent PCI and CABG, respectively. In the PCI cohort, mean delta SYNTAX score was 13.9±10.0 and mean rSS was 8.8±8.2 (Figure 2). Study patients were divided into three groups: rSS≤12 group (n=445), rSS>12 group (n=150) and CABG group (n=448). Table 1 presents the baseline clinical, angiographic and medical characteristics, stratified by rSS after PCI or CABG. Compared with the rSS>12 group, the rSS≤12 group had a lower prevalence of dyslipidaemia and previous MI. Compared with the CABG group, the rSS≤12 group had a lower prevalence of diabetes mellitus and previous MI. The left ventricular ejection fraction was significantly different between the rSS≤12 group and the CABG group (58.0±12.0 vs. 52.8±13.9, p<0.01). In terms of angiographic characteristics, the rSS≤12 group had a lower prevalence of right coronary artery (RCA)-CTO, multiple CTOs, calcified CTO and proximal-to-mid CTO than the rSS>12 group. Baseline SYNTAX score was lower in the rSS≤12 group than in the rSS>12 group (20.6±8.5 vs. 26.1±7.8, p<0.01). Compared with the CABG group, the rSS≤12 group had a lower prevalence of RCA-CTO, multiple CTOs, bridge collaterals, calcified CTO, and proximal-to-mid CTO. Baseline SYNTAX score was lower in the rSS≤12 group than in the CABG group (20.6±8.5 vs. 30.3±10.3, p<0.01). In terms of concomitant medication after revascularisation, clopidogrel was received more frequently in the rSS≤12 group than in the rSS>12 group. The rSS≤12 group more frequently took clopidogrel than the CABG group, though the rSS≤12 group took statins and beta-blockers less regularly (Table 1).
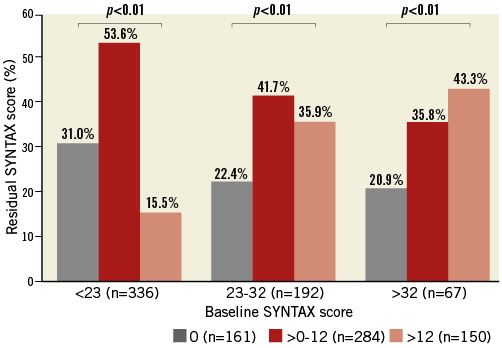
Figure 2. Complete (residual SYNTAX score 0), reasonable incomplete (0
CLINICAL OUTCOMES
The median follow-up duration was 42 months (interquartile range 22-68 months). Table 2 presents the cumulative clinical outcomes of the three study groups. Cardiac death occurred in 14 patients (3.1%) in the rSS≤12 group, 14 patients (9.3%) in the rSS>12 group (p<0.01 for comparison with the rSS≤12 group) (Figure 3A), and 29 patients (6.5%) in the CABG group (p=0.04 for comparison with the rSS≤12 group) (Figure 3B). All-cause death occurred in 33 patients (7.4%) in the rSS≤12 group, 23 patients (15.3%) in the rSS>12 group (adjusted p<0.01) (Figure 3C), and 59 patients (13.2%) in the CABG group (adjusted p=0.02) (Figure 3D). In multivariate Cox regression analysis, the rSS≤12 group had a lower risk of cardiac death (adjusted hazard ratio [HR] 0.35, 95% confidence interval [CI]: 0.16 to 0.75; p=0.01) and all-cause death (adjusted HR 0.50, 95% CI: 0.29 to 0.86; p=0.01) than the rSS>12 group after adjustment for prevalence of multiple CTOs, previous MI, proximal-to-mid CTO and RCA-CTO. The rSS≤12 group had a similar risk of cardiac death (adjusted HR 0.63, 95% CI: 0.32 to 1.23; p=0.17) and all-cause death (adjusted HR 0.67, 95% CI: 0.43 to 1.05; p=0.08) compared with the CABG group, after adjustment for prevalence of diabetes mellitus, multiple CTOs, previous MI, proximal-to-mid CTO and RCA-CTO. The incidence of repeat revascularisation was higher in the rSS≤12 group than in the CABG group (12.6% versus 0.9%, adjusted HR 17.7, 95% CI: 6.28 to 49.74; p<0.01) (Table 2).
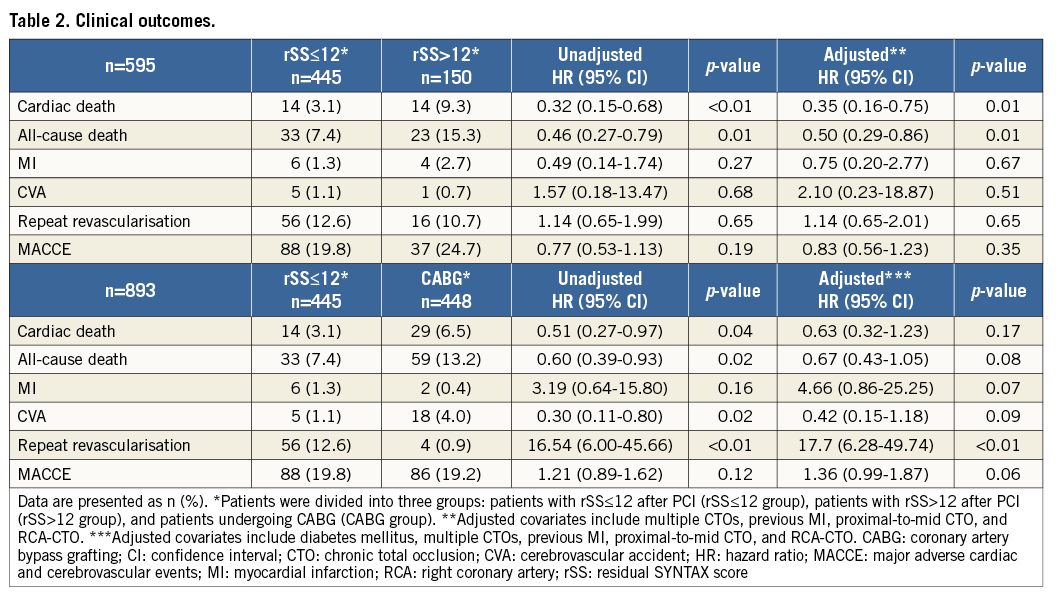
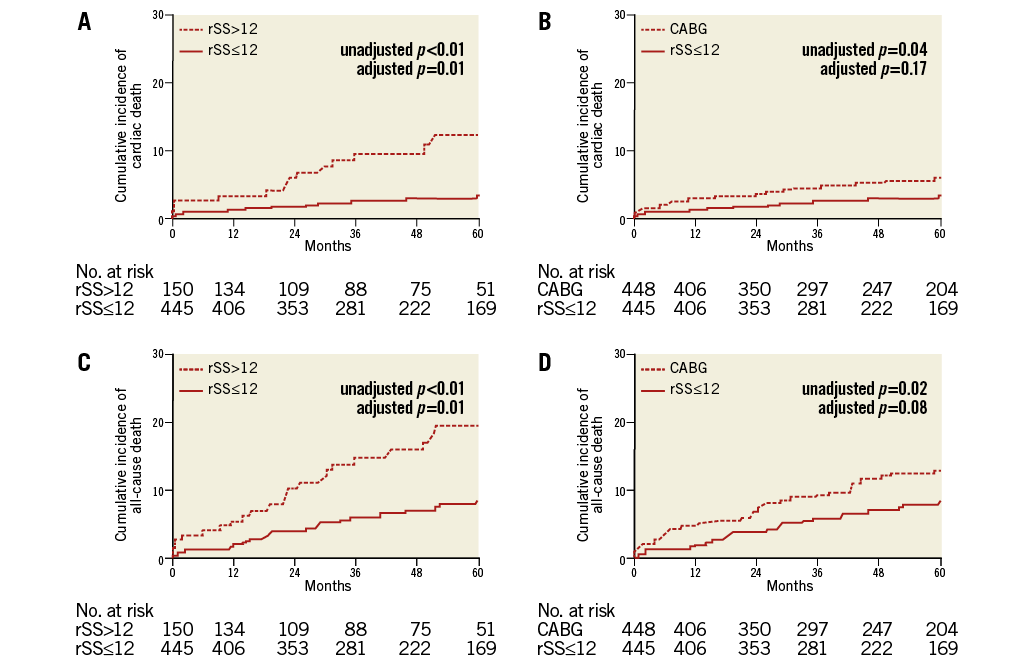
Figure 3. Kaplan-Meier curves for clinical outcomes. A) Kaplan-Meier curves for cardiac death in patients with an rSS≤12 compared to those with an rSS>12 after PCI. B) Kaplan-Meier curves for cardiac death in patients with an rSS≤12 after PCI compared to those undergoing CABG. C) Kaplan-Meier curves for all-cause death in patients with an rSS≤12 compared to those with an rSS>12 after PCI. D) Kaplan-Meier curves for all-cause death in patients with an rSS≤12 after PCI compared to those undergoing CABG.
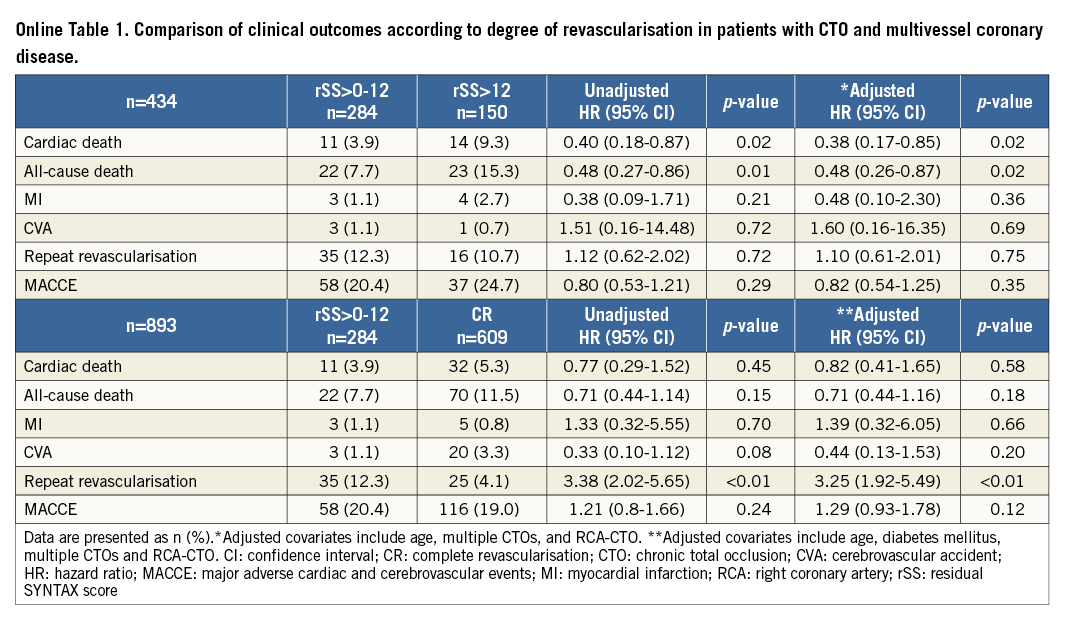
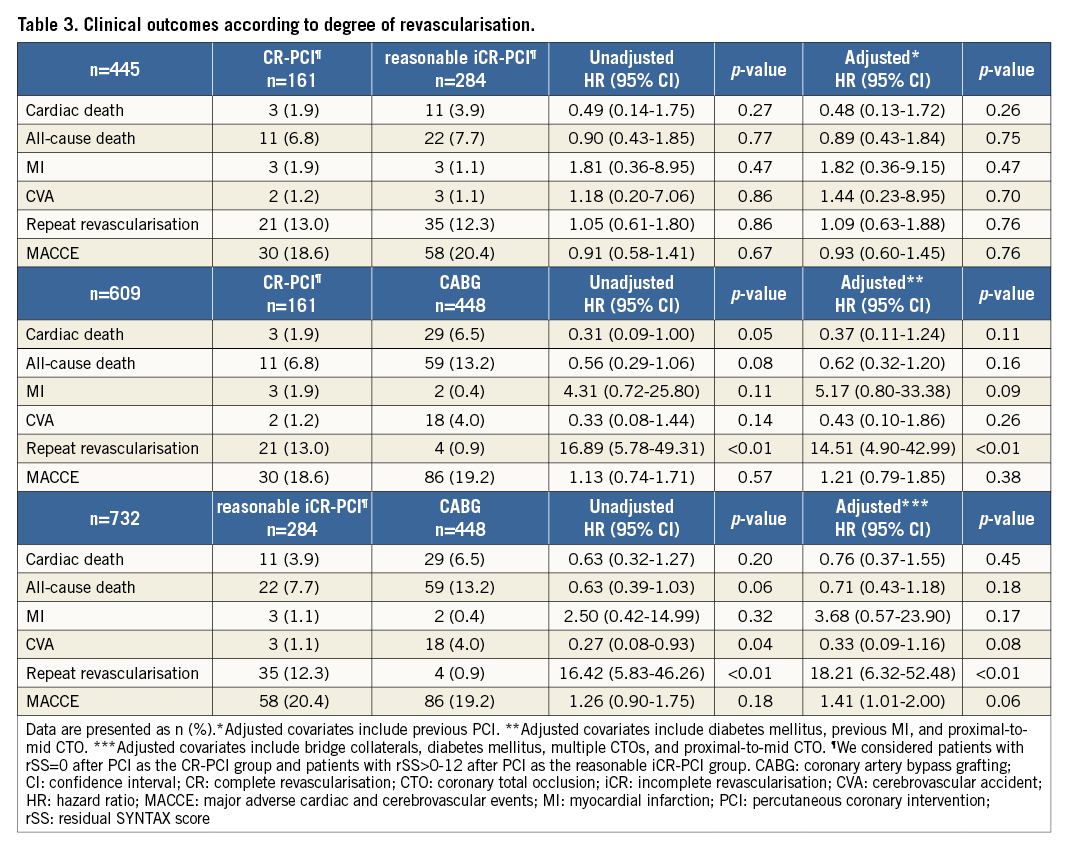
COMPARISON OF CLINICAL OUTCOMES ACCORDING TO THE DEGREE OF REVASCULARISATION
We evaluated clinical outcomes according to the degree of revascularisation. Patients in the study were re-divided into four subgroups: rSS=0 (complete revascularisation [CR]-PCI subgroup), rSS>0-12 (reasonable incomplete revascularisation [iCR]-PCI subgroup), rSS>12 (iCR-PCI subgroup) and the CABG group (Online Figure 1). In multivariate Cox regression analysis, the CR-PCI subgroup, the reasonable iCR-PCI subgroup and the CABG group had a significantly lower risk of cardiac death and all-cause death than the iCR-PCI subgroup without discrimination (Figure 4 and Online Table 1). The CR-PCI subgroup had a similar risk of cardiac death and all-cause death compared with the reasonable iCR-PCI subgroup, after adjustment for previous PCI. The CR-PCI subgroup had no different incidences of cardiac death and all-cause death compared with the CABG group, after adjustment for diabetes mellitus, previous MI, and proximal-to-mid CTO. The reasonable iCR-PCI subgroup also had a similar risk of cardiac and all-cause mortalities after adjustment for bridge collaterals, diabetes mellitus, multiple CTOs and proximal-to-mid CTO. The incidence of repeat revascularisation was higher in the reasonable iCR-PCI subgroup and the CR-PCI subgroup than in the CABG group (Table 3).
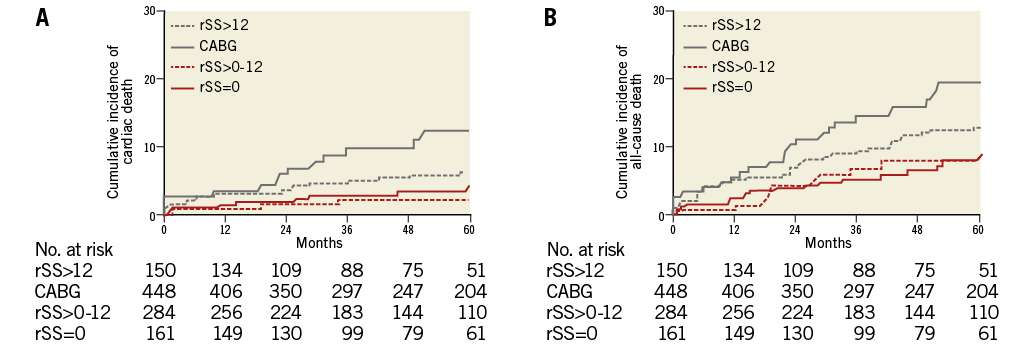
Figure 4. Kaplan-Meier curves for clinical outcomes according to degree of revascularisation. Patients in the present study were divided into four subgroups: rSS=0, rSS>0-12 , rSS>12 and CABG. Kaplan-Meier curves for cardiac death (A) and for all-cause death (B).
Discussion
We investigated the reasonable degree of incomplete revascularisation and the discriminatory power of rSS for risk reduction of cardiac mortality in patients with CTO and multivessel CAD. The main finding of our study is as follows: patients with an rSS≤12 had significantly lower cardiac mortality than those with an rSS>12 after PCI and exhibited cardiac mortality comparable to subjects who underwent CABG. Although this study was limited by its observational design, this is the first report specifically addressing quantification of residual coronary stenosis and reasonable level of incomplete revascularisation using rSS in patients with CTO and multivessel CAD. Moreover, the survival benefit related to the reasonable degree of incomplete revascularisation after CTO-PCI was acceptable in these patients.
Previous studies have shown a survival benefit in CTO patients who underwent successful PCI compared to those who underwent failed procedures1,14. However, it is often difficult to achieve CR in patients with CTO, and doing so may have potentially lethal complications. Moreover, it is difficult to establish whether an intervention is adequate and the prognostic value of quantifying the extent and complexity of residual coronary stenosis has not been demonstrated. The SYNTAX trial recruited subjects with substantially more complex CAD (left main or de novo three-vessel coronary disease, median SYNTAX score 28, interquartile range 20.0-36.0 months). Farooq et al validated the observation that an rSS>8 is associated with adverse outcomes and is a powerful indicator of five-year mortality5. Généreux et al7 also suggested that an rSS<8 had adequate discriminatory power for risk prediction of one-year ischaemic outcomes after PCI. Based on these investigations, rSS may aid in the determination of a reasonable level of revascularisation in patients with de novo multivessel CAD. However, comparison of outcomes using the original rSS of 8 yielded no conclusive results in our study. High frequencies of failed PCI and perioperative complication related to CTO revascularisation still exist. Moreover, patients with CTO might tolerate a higher degree of incomplete revascularisation because of collateral circulation. Therefore, we evaluated a reasonable incomplete revascularisation strategy in patients with at least one CTO and multivessel CAD using the rSS scoring system, a useful tool for stratifying the extent and complexity of residual coronary stenosis after CTO-PCI. In our study, patients with an rSS≤12 demonstrated survival benefit compared with patients with an rSS>12 after CTO-PCI. Patients with an rSS≤12 had significantly lower cardiac and all-cause mortality rates than those with an rSS>12, and had mortality rates comparable to subjects with CABG. In addition, patients with rSS>0-12 had a similar risk of cardiac and all-cause mortality compared to rSS=0 or CABG patients. The difference in degree of rSS between previous studies (rSS<8) and our study (rSS≤12) may be related to differences in the patient characteristics of those with stable non-occlusive CAD versus CTO. Further reducing ischaemic burden would undoubtedly improve long-term prognosis, as exemplified by previous studies in patients with stable angina who were ultimately revascularised15. However, because of the possible lethal complications and lower procedural success rates related to CTO-PCI, CTO revascularisation needs to be considered more carefully. Although the reason for this difference in cut-off values is unclear, distal coronary collaterals (which are frequently present in CTO lesions with a catheterisation-documented incidence of 80 to 90%) may lead to protection of myocardium vulnerable to ischaemia, resulting in improved survival rates in patients with CTO16,17. Therefore, an rSS≤12 could be an indicator of lower long-term cardiac mortality, and may aid in the determination of a reasonable level of revascularisation in patients with CTO and multivessel CAD.
Study limitations
This study has several limitations. First, its design was non-randomised, retrospective, and observational, which may have significantly affected the results due to confounding factors. In addition, it might be difficult to generalise clinical outcomes between the CABG and PCI groups because patients undergoing CABG had different characteristics compared to patients treated with PCI. Second, due to the retrospective nature of our registry, data regarding preprocedural and post-procedural medications and changes in medical therapies during follow-up were not always available. Third, we did not routinely evaluate the amount of viable myocardium or ischaemia using functional ischaemia testing, nor did we assess graft flow or distal perfusion with follow-up coronary angiography in patients who underwent CABG. Fourth, we observed high rates of complete arterial graft and successful PCI in our hospital and cannot exclude the possibility of under-reporting of clinical outcomes other than death or the underestimation of rSS in patients treated with CABG. Finally, we established the 12 score as the rSS cut-off value, not the 8 score in the original SYNTAX trial or the best ROC cut-off value in our data. Unfortunately, because of the retrospective nature of our registry, we could not explain the origin of these differences or the mechanism of the protective effect for myocardium vulnerable to ischaemia exerted by collateral flow. However, CTO patients may tolerate a higher degree of iCR and only an rSS>12 after PCI was associated with poor outcomes.
Conclusions
In patients with CTO and multivessel CAD, patients with an rSS≤12 had significantly lower cardiac mortality compared to those with an rSS>12 after PCI, and demonstrated cardiac mortality comparable to subjects who underwent CABG. Based on our results, treatment of patients with an rSS≤12 after PCI may reduce the risk of cardiac mortality. This cut-off could be used as a measure of reasonable incomplete revascularisation in patients with CTO and multivessel CAD. A large-scale, randomised trial is needed to confirm these findings.
| Impact on daily practice Little is known about reasonable incomplete revascularisation after PCI. This is the first report specifically addressing this issue using rSS in patients with CTO and multivessel CAD. Our results suggest that reasonable incomplete revascularisation with treatment of rSS≤12 after PCI may be attainable, and that an rSS≤12 could aid in determining a reasonable level of revascularisation in these patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Online Table 1. Comparison of clinical outcomes according to degree of revascularisation in patients with CTO and multivessel coronary disease.
Online Figure 1. Scheme of subgroup distribution. CABG: coronary artery bypass grafting; CTO: chronic total occlusion; PCI: percutaneous coronary intervention; rSS: residual SYNTAX score