
Abstract
Aims: The success of drug-coated balloon therapy might be compromised by intraprocedural particulate embolisation of matrix coating, which may cause downstream microvascular obstruction. We aimed to investigate whether drug-coated balloon therapy was associated with an increase in markers of myocardial necrosis compared with drug-eluting stents or plain balloon angioplasty.
Methods and results: In the ISAR-DESIRE-3 trial, patients with limus-eluting stent restenosis were randomised to treatment with a paclitaxel-coated balloon (PCB), paclitaxel-eluting stent (PES) or balloon angioplasty. We included enrolled patients who had pre- and post-intervention high-sensitivity troponin (hs-TnT) levels available. The delta (peak post-procedure minus baseline) troponin was compared between treatment arms. The association between delta troponin tertiles and three-year mortality was also evaluated. Three hundred and forty-three (343) patients were included, comprising patients treated with PCB (n=115), PES (n=112) and balloon angioplasty (n=116). Groups were well matched in terms of baseline characteristics. There was no difference in delta troponin in patients treated with PCB, PES and balloon angioplasty (36±65, 70±183, and 51±124 ng/L, respectively, p=0.16). Three-year mortality was 7.7%, 8.8% and 14.3% for the 1st, 2nd and 3rd tertiles of delta troponin, respectively, p=0.23.
Conclusions: In patients with drug-eluting stent restenosis, there was no difference in subclinical myocardial necrosis among patients treated with PCB, PES, and balloon angioplasty. ClinicalTrials.gov Identifier: NCT00987324
Abbreviations
CFR: coronary flow reserve
DCB: drug-coated balloon
DES: drug-eluting stent
hs-TnT: high-sensitivity troponin T
ISR: in-stent restenosis
PCB: paclitaxel-coated balloon
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
Introduction
Drug-coated balloons (DCB) have demonstrated efficacy in the treatment of restenosis within bare metal stents and drug-eluting stents (DES), with a class I, level of evidence A recommendation for use for the treatment of these conditions in current European guidelines1. At present, paclitaxel remains the only clinically proven antirestenotic agent used in DCB technology. As paclitaxel is a hydrophobic, highly crystalline agent, it is mixed with a hydrophilic excipient or spacer to improve its solubility and prevent clumping of particles on the balloon surface. This aims to minimise loss of drug during transit to the target lesion (termed “floating time”) and to facilitate its uptake into the arterial wall2.
Preclinical studies of paclitaxel-coated balloons (PCB) in porcine coronary artery models using a variety of different matrix preparations showed that only small proportions of the coating are taken up by the vessel wall or remain on the balloon surface, with much of the drug coating being unaccounted for3,4. Moreover, examination of downstream microvascular beds in preclinical studies occasionally reveals distal embolisation of microparticles of matrix coating5. In clinical settings, this could conceivably translate into an increased risk of microvascular ischaemia. Indeed, a clinical study of coronary flow reserve (CFR) after DCB therapy showed a transient reduction in CFR of unclear aetiology immediately after DCB angioplasty6.
Against this background, we investigated whether differences in myocardial necrosis could be detected after coronary DCB angioplasty compared with plain balloon angioplasty or DES using high-sensitivity markers of myocardial damage.
Methods
STUDY POPULATION, DEVICES, AND INTERVENTION PROTOCOL
Patients enrolled in the Intracoronary Stenting and Angiographic Results: Drug Eluting Stent In-Stent Restenosis: 3 Treatment Approaches (ISAR-DESIRE-3) trial who had baseline (preprocedural) and post-procedural high-sensitivity troponin T (hs-TnT) measurements available for analysis were included in the current study.
The trial protocol has been described in detail previously7. In brief, patients were eligible if they were >18 years of age with symptoms or objective evidence of myocardial ischaemia in the presence of restenosis of ≥50% located in a native vessel DES or its proximal or distal margins, provided that written, informed consent for participation in the study was obtained from the patient or her/his legally authorised representative. Patients with restenosis occurring in any limus-eluting drug-eluting stent, including DES eluting sirolimus or its analogues, were considered eligible for participation in the study. Exclusion criteria included target lesion location in the left main stem or in a coronary bypass graft, ST-elevation myocardial infarction within the preceding 48 hours, cardiogenic shock, severe renal insufficiency (defined as glomerular filtration rate ≤30 ml/min), and any comorbid condition resulting in life expectancy of less than 12 months or that may result in protocol non-compliance.
Patients were assigned in a 1:1:1 allocation to treatment with an open-label PCB (SeQuent Please®; B. Braun, Melsungen, Germany), paclitaxel-eluting stent (PES) (TAXUS™ Liberté™; Boston Scientific, Marlborough, MA, USA), or balloon angioplasty. The PCB catheter is coated with 3 µg of paclitaxel per square millimetre of balloon surface using iopromide as a hydrophilic excipient. Stenting was strongly discouraged in the PCB and balloon angioplasty treatment groups. Indications for stenting in the angioplasty arms were large dissections, particularly with flow limitation, as well as residual stenosis >50% after multiple balloon dilations.
An oral loading dose of platelet adenosine diphosphate (ADP) receptor antagonist was administered to all patients prior to the intervention. During the procedure, patients were given intravenous aspirin and heparin, with or without glycoprotein inhibitors or bivalirudin. After the intervention, all patients, regardless of treatment allocation, were prescribed 200 mg/day aspirin indefinitely, and an oral platelet ADP receptor antagonist for at least six months. After randomisation, blood samples for the determination of cardiac biomarkers (CK, CK-MB, hs-TnT) were drawn every eight hours for the first 24 hours and daily thereafter. Daily ECG recording was also performed until discharge. Patients were evaluated at one, 12 and 36 months by phone or office visit.
BIOCHEMICAL MEASUREMENTS
Blood samples were collected in tubes containing a lithium-heparin anticoagulant. Troponin T was measured using a high-sensitivity assay in a cobas e 411 immunoanalyser based on electrochemiluminescence technology (Roche Diagnostics, Rotkreuz, Switzerland). The limit of blank for this assay –the concentration below which analyte-free samples are found with a probability of 95% – is ≤3 ng/L. The functional sensitivity – the lowest analyte concentration that can be reproducibly measured with a coefficient of variation ≤10% – is ≤13 ng/L. The 99th upper reference limit is 14 ng/L. Baseline and peak post-procedural hs-TnT were used for the current analysis. Other biochemical parameters were measured using standard laboratory methods by laboratory personnel unaware of patient clinical or follow-up data.
DATA MANAGEMENT, ENDPOINTS, AND DEFINITIONS
Data were collected and entered into a computer database in the Clinical Data Management Centre (ISAResearch Centre, Munich, Germany) by specialised personnel. All events were adjudicated and classified by an event adjudication committee blinded to the treatment groups.
The primary endpoint was delta troponin, defined as the magnitude of change between baseline and peak post-procedural troponin levels, was calculated by subtracting the baseline from the peak post-procedural levels, and compared between the treatment groups. The secondary endpoint of interest was three-year all-cause mortality according to magnitude of delta troponin by tertile (irrespective of the treatment received). Myocardial infarction adjudication was on the basis of clinical symptoms, ECG and cardiac biomarkers, and has been previously described in detail8. Acute coronary syndrome was defined as unstable angina pectoris or ST-segment or non-ST-segment elevation myocardial infarction. Academic Research Consortium criteria for definite stent thrombosis were used for adjudication of target lesion thrombosis9.
STATISTICAL ANALYSIS
Only patients with available baseline, preprocedural and peak, post-procedural hs-TnT values were included in the statistical analysis. The study population was also divided into tertiles according to magnitude of delta troponin, irrespective of the treatment received. Continuous data are reported as mean (SD) or median (25th-75th percentiles). Categorical data are presented as counts or proportions (%). Differences across groups at baseline were checked for significance using analysis of variance (ANOVA) for continuous data and the chi-squared test (or Fisher’s exact test where the expected cell value was <5) for categorical variables. Categorical variables were tested across groups, with pairwise testing only in cases of significant differences across groups, using the Student’s t-test for continuous data and the chi-squared test (or Fisher’s exact test where the expected cell value was <5) for categorical variables to check for significance. Survival was assessed using the Kaplan-Meier method and compared using the log-rank test. Statistical software S-PLUS, version 4.5 (S-PLUS®; Insightful Corporation, Seattle, WA, USA) was used for all other analyses. Data analysis was performed by R.A. Byrne and A. Kastrati.
Results
ANALYSIS OF DELTA TROPONIN ACCORDING TO TREATMENT GROUP
Three hundred and forty-three patients with in-stent restenosis (ISR) in a limus-eluting stent randomised to treatment with PCB (n=112), PES (n=116) or balloon angioplasty (n=115) had available pre- and post-procedure hs-TnT measurements. There were no significant differences in terms of baseline patient characteristics (Table 1). Sixty-seven (20.0%) patients presented with acute coronary syndrome at the time of enrolment.
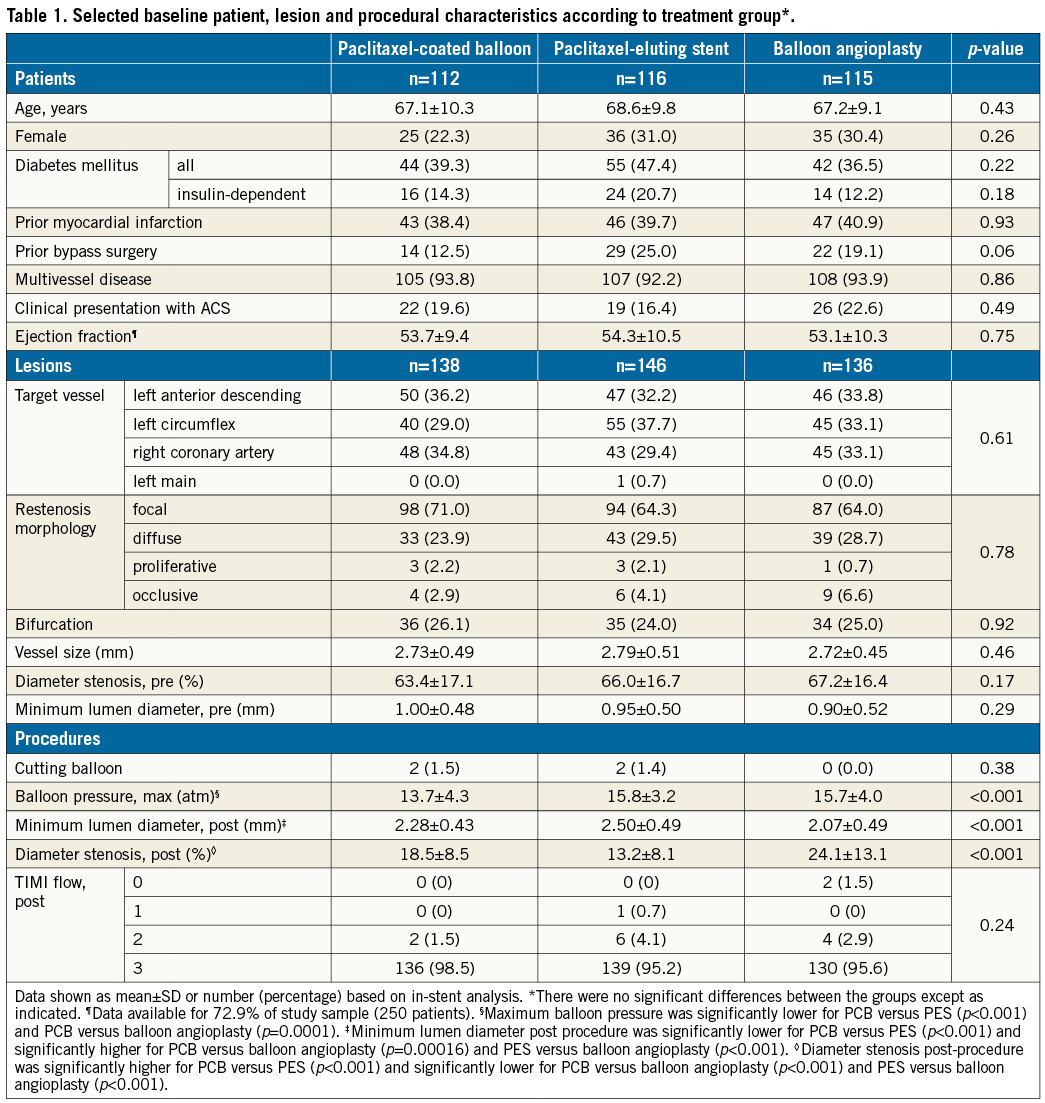
A total of 420 lesions were treated with PCB (n=138), PES (n=146) or balloon angioplasty (n=136). Angiographic and procedural characteristics are displayed in Table 1. At the time of enrolment, focal pattern in-stent restenosis was present in 279 (66.4%) lesions.
There were no differences among the groups in terms of the proportion of patients treated as per protocol. Nine lesions in the PCB group were treated with stent implantation; 10 lesions in the PES group were treated with balloon dilatation only; eight lesions in the balloon angioplasty group were treated with stent implantation, p=0.95.
There was no significant difference between PCB, PES and balloon angioplasty in terms of mean delta troponin (36±65, 70±183, and 51±124 ng/L, respectively, p=0.16). Cumulative frequency distribution curves displaying the distribution of delta troponin according to treatment group are shown in Figure 1.
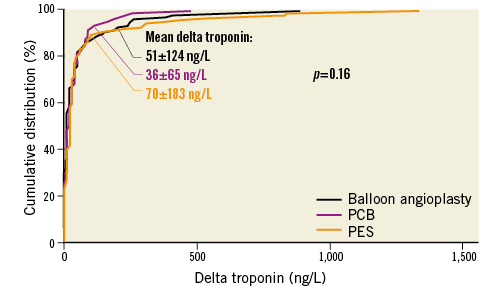
Figure 1. Cumulative distribution curves showing delta troponin according to treatment group.
There was no significant difference among treatment groups in terms of TIMI flow grade post intervention or the proportion of patients with a peak hs-TnT value more than five times the 99th percentile upper reference limit of normal: PCB: 24 (30.4%); PES: 29 (36.7%); and balloon angioplasty: 26 (32.9%) patients, p=0.81. When analysed per protocol, mean delta troponin levels were as follows: PCB=34±64; PES=67±182; and balloon angioplasty=42±92 ng/L, p=0.13.
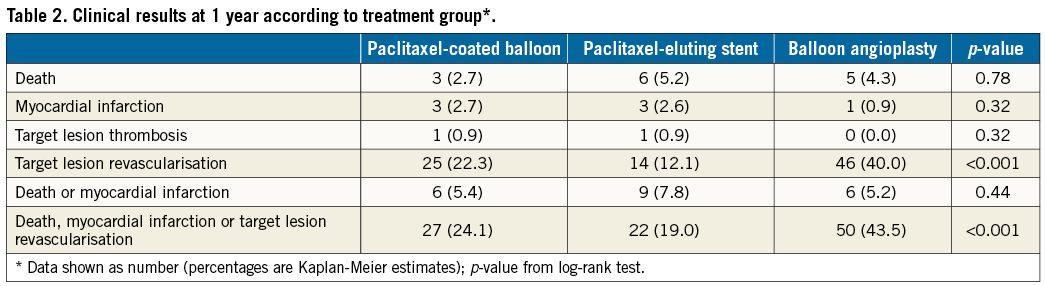
Clinical outcomes at one year according to treatment group are shown in Table 2.
SURVIVAL ANALYSIS ACCORDING TO TERTILES OF DELTA TROPONIN
Patients were also categorised according to tertiles of delta troponin. Mean delta troponin values per tertile were 0±8, 15±5 and 132±197 ng/L, respectively, p<0.001. There were no significant differences in terms of baseline patient, lesion, or procedural characteristics (Table 3), with the exception of a higher mean maximum balloon pressure with each increase in tertile of delta troponin and a trend towards a higher proportion of bifurcation lesions in the third tertile.
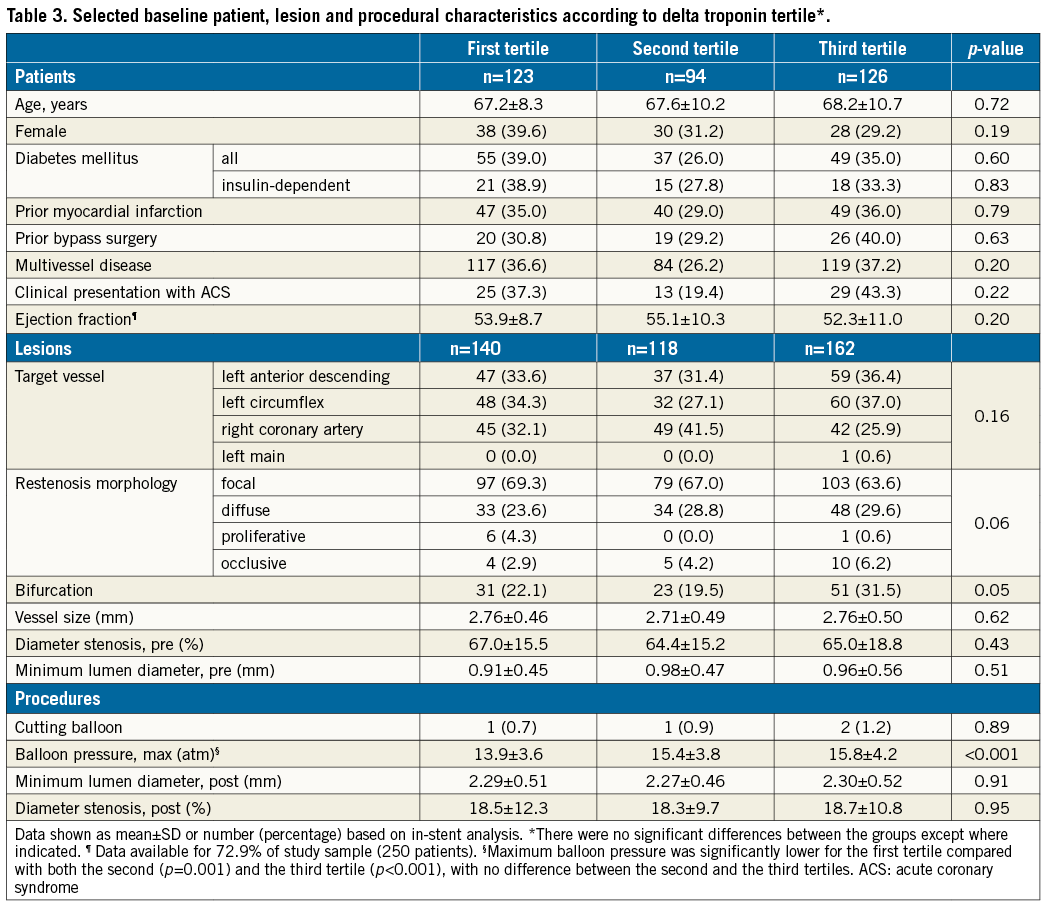
Mean baseline hs-TnT was 22±45, 13±31, and 40±88 ng/L (p=0.005) in the first, second and third tertiles of delta troponin, respectively. Respective mean peak hs-TnT was 23±39, 28±33, and 172±240 ng/L (p<0.001). Mean peak CK-MB level remained within normal limits in each tertile, at 15.1±8.7, 15.2±6.0, and 21.4±12.7 U/L, respectively (p<0.001). Mean C-reactive protein was 3.7±5.0, 2.5±2.8, and 5.5±9.2 mg/L, respectively (p=0.05). Mean baseline creatinine level was 1.19±1.16, 1.07±0.89, and 1.18±0.83 mg/dL, respectively (p=0.62).
Three-year mortality rates according to delta troponin tertile were 7.7%, 8.8% and 14.3% for the first, second and third tertiles, respectively (p=0.23) (Figure 2).
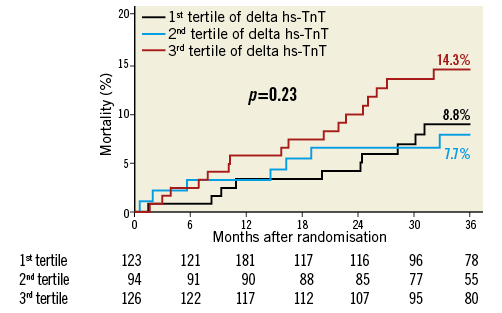
Figure 2. Kaplan-Meier curves showing mortality rate according to delta troponin tertile. hs-TnT: high-sensitivity troponin T
Discussion
The main finding of this report is that, in patients with ISR within a limus-eluting stent, treatment with a PCB was not associated with a greater troponin rise or reduction in TIMI flow grade compared to treatment with DES or balloon angioplasty. Furthermore, in patients with a troponin rise, irrespective of the therapy received, the magnitude of rise did not appear to correlate with mortality risk at three years.
DCB angioplasty may be associated with a risk of distal embolisation of particulate balloon coating, consisting of antirestenotic drug and excipient5. Preclinical studies of PCB catheters in porcine coronary overstretch models, using a variety of different matrix formulations, comprising urea, iopromide and acetone excipients mixed with paclitaxel at concentrations of 3.0, 3.0 and 2.5 µg/mm2, respectively, reported that approximately 6% of the drug coating is lost during floating time, 10-30% is taken up by the vessel wall during balloon inflation, and up to 10% remains on the balloon3,4. The fate of the remainder of the drug coating is unknown but distal embolisation has been proposed as a potential explanation. Indeed, during preliminary coating development, an early DCB-coating formulation was abandoned on account of unacceptable distal embolic findings10.
Particulate embolisation in the coronary arteries may result in impaired flow or periprocedural myocardial infarction5. Slow flow or no reflow immediately after DCB angioplasty of ISR lesions has occasionally been observed and hypothesised to be due to distal embolisation of DCB coating11,12. Moreover, a clinical study of CFR after DCB therapy showed a transient reduction in CFR of unclear aetiology immediately after DCB angioplasty (using the IN.PACT® DCB [Medtronic, Dublin, Ireland], coated with 3.0 µg/mm2 paclitaxel and a urea excipient), which resolved spontaneously within ten minutes6. The authors concluded that distal embolisation in the microvascular bed was the most likely explanation given the major contribution of the coronary microcirculation to CFR6.
In patients with peripheral artery disease undergoing angioplasty with DCB, there have also been some safety concerns possibly attributable to distal embolisation of particulate coatings2. The IN.PACT DEEP randomised trial compared treatment of infrapopliteal peripheral artery disease with plain balloon angioplasty versus DCB therapy using the IN.PACT Amphirion balloon for below-the-knee indications13. Although the trial formally met its non-inferiority hypothesis with regard to the primary safety endpoint, there was an unexplained trend towards more major amputation in the DCB arm (8.8% vs. 3.6%; p=0.08). This finding ultimately led to market withdrawal of the study device. In contrast, results of randomised studies examining other iterations of the IN.PACT DCB technology in the femoropoliteal territory (namely the IN.PACT Pacific™ and IN.PACT Admiral™ DCB devices), as well as other DCB did not raise such concerns. One potential explanation for these observed differences is the differing balloon-coating processes for these devices. In particular, the IN.PACT Amphirion DCB was coated after wrapping of the balloon, rather than beforehand, as for other DCB devices. This may have resulted in non-uniform drug coating on the balloon surface, with all of the matrix coating on the outer exposed surface, rendering it vulnerable to loss during transit to, and during deployment at the target lesion2.
A prior preclinical study examined angiographic or histopathological evidence of distal embolisation after low-pressure inflation of the Lutonix® DCB (polysorbate/sorbitol excipient and 2.0 µg/mm2 paclitaxel; Bard Peripheral Vascular, Tempe, AZ, USA) against a balloon angioplasty control in a swine femoral artery model at 28, 90 and 180 days post procedure. Although no evidence of vascular embolisation or dependent tissue ischaemia downstream from the intervention was found, it is important to take into consideration the variable coating integrity reported among DCB devices. For example, less than 0.1% of the drug coating was found to be lost during inflation and dry shaking of the DCB used in the study in question10. On the other hand, 20-40% paclitaxel loss has been reported for DCB with urea and iopromide excipients3. This would imply that the microstructure of the DCB surface coating – including presence or absence of a spacer, choice of spacer, and concentration of both paclitaxel and spacer – is also likely to be important in promoting or limiting downstream particulate embolisation5.
Interestingly, a previous clinical study comparing patients treated with PCB (also the SeQuent® Please device; B. Braun, Melsungen, Germany) and PES in the setting of de novo lesions in stable CAD found significantly lower proportions of patients with peak post-procedural troponin T levels more than five times the 99th percentile upper reference limit of normal within 48 hours of percutaneous coronary intervention (PCI) in the PCB compared with the PES group14. This finding contrasts with that of the current study: although mean delta troponin was numerically lower in the PCB compared with the PES group in our study, this difference was not statistically significant. Moreover, the proportion of patients in whom peak hs-TnT exceeded five times the 99th percentile upper reference limit of normal was similar across treatment groups. The differences between the two studies should be interpreted in the context of the non-randomised nature of the prior study, its consecutive cohort design and the small sample size (52 patients in each group).
In light of this evidence, our analysis offers some reassurance in relation to myocardial injury after DCB angioplasty. Although prior randomised controlled trials failed to detect differences in the rates of myocardial infarction between patients treated with DCB or repeat stenting7,15-17, our analysis is the first to report on detailed analysis of hs-TnT following intervention. In this respect, the lack of difference observed across the treatment groups speaks against a relevant difference in subclinical myocardial injury and supports the safety of DCB use for this indication.
We also examined the association between the delta troponin levels observed and survival of patients at three-year follow-up. Despite a marked difference in delta troponin between patients in the third tertile (132±197 ng/L) compared with those in the first (0±8 ng/L) and second (15±5 ng/L) tertiles, we found no significant difference in three-year mortality rates by delta troponin tertile. While numeric differences were observed with mortality rate increasing with increasing tertile of troponin, these differences were not statistically different. Although the power of our study to detect differences in mortality is limited by the relatively small sample size, this observation is broadly in line with larger studies showing that, while baseline elevated hs-TnT level is an independent predictor of mortality in patients undergoing PCI18,19, delta hs-TnT adds little prognostic information beyond what is already known from the baseline hs-TnT level20.
In summary, our report may be of interest as it represents the first dedicated study looking for evidence of myocardial necrosis due to distal embolisation following DCB angioplasty using a high-sensitivity troponin assay. Moreover, use of delta hs-TnT rather than peak value as a marker of procedure-related hs-TnT rise avoids the confounding effect of baseline hs-TnT.
Limitations
Our study has a number of important limitations. Firstly, only patients with restenosis occurring within limus-eluting DES were enrolled. These data cannot be applied to patients undergoing intervention of de novo lesions or for restenosis within other stent types. Secondly, our study used a PCB based on an iopromide excipient. As significant differences exist between different DCB catheters, the results of our study may not be generalisable to other devices. However, in preclinical models, iopromide-based DCB showed the lowest coating integrity, so DCB employing this excipient may carry a relatively higher risk of distal embolisation compared with those based on alternative excipients. Thirdly, measurement of cardiac biomarkers is an indirect way of measuring effects of distal embolisation in coronary vasculature. Alternative methods, such as systematic cardiac magnetic resonance imaging might offer additional insight regarding downstream effects of embolisation, though may not be sensitive enough to detect differences between groups given the small magnitude of troponin change seen in this study. Finally, this study was not powered to detect differences in clinical endpoints between troponin tertiles, meaning that lack of a difference in three-year mortality between tertiles does not reliably rule one out.
Conclusions
Treatment of DES ISR with a paclitaxel DCB as compared to repeat DES implantation or balloon angioplasty was not associated with increased myocardial necrosis as evidenced by a post-procedural rise in high-sensitivity troponin T. Moreover, the level of change in high-sensitivity troponin was low across the treatment groups.
| Impact on daily practice Despite evidence of distal particulate embolisation of DCB matrix coating in preclinical studies, we observed no biomarker evidence of clinical or subclinical downstream microvascular ischaemia in the clinical setting. This is a reassuring finding, supporting the safety of DCB for the treatment of coronary ISR. |
Guest Editor
This paper was guest edited by Fernando Alfonso, MD, PhD; Hospital Universitario de La Princesa, Universidad Autónoma de Madrid, Madrid, Spain.
Funding
Study design, analysis and funding were provided by Deutsches Herzzentrum München, Munich, Germany. No extramural funding was used in the conduct of this trial.
Conflict of interest statement
R. Colleran reports receiving support from the Irish Board for Training in Cardiovascular Medicine sponsored by MSD. M. Joner reports having received speaker fees from Biotronik, Boston Scientific, Medtronic, and OrbusNeich, as well as consultant fees from Biotronik. A. Kastrati reports patents issued related to drug-eluting stent coatings. R. Byrne reports receiving institutional research grants from Boston Scientific and HeartFlow and lecture fees from B. Braun, Biotronik and Boston Scientific. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

