
Abstract
Aims: The aim of this study was to investigate relocation of minimal lumen area (MLA) after implantation of a bioresorbable scaffold (BRS).
Methods and results: In the ABSORB II randomised trial (BRS vs everolimus-eluting stent [EES]), lesions were investigated by serial intravascular ultrasound pre procedure, post procedure, and at three years. MLA relocation was defined as an axial MLA shift of more than 2.4 mm. MLA relocation from post procedure to three years was observed in 163/237 (68.8%) and 75/129 (58.1%) of lesions treated by BRS and EES, respectively (p=0.041). When matching preprocedural MLA site with the same topographical sites post procedure and at three years, BRS showed significant late lumen enlargement and expansive remodelling compensating for significant plaque increase, whereas EES showed significant late lumen narrowing with significant plaque growth not compensated for by expansive remodelling from post procedure to three years. In the multivariate analysis, female gender, previous PCI, BRS implantation, total device length, and maximal pressure (either at device implantation or post-dilatation) were independently associated with MLA relocation from post procedure to three years.
Conclusions: MLA relocation from post procedure to three years was more frequent in BRS than EES. Late lumen enlargement and expansive vessel remodelling at the preprocedural MLA site was observed in BRS, but not in EES.
Introduction
Late loss, which is the difference between post-procedure and follow-up minimal luminal diameter (MLD) measured by quantitative coronary angiography (QCA), has been one of the most commonly accepted parameters to assess the efficiency of balloon angioplasty, bare metal stents, drug-eluting stents, and bioresorbable scaffolds (BRS). However, late loss is calculated regardless of the respective axial location of the MLDs along the treated area post procedure and at follow-up; therefore, it does not necessarily reflect the actual vessel wall change at the site of the original MLD/MLA.
Intuitively, when we analyse serial investigation of minimal lumen area (MLA) obtained with intravascular ultrasound, we assume that the decrease (or increase) in MLA occurs at the initial location of narrowing post procedure. The statistical reports of serial assessment of MLA post procedure and at follow-up are sometimes misinterpreted, since the statistical evaluation (paired values) suggests that the same longitudinal region of interest or the same cross-section(s) have been compared. Long-term outcome by intravascular ultrasound (IVUS) or angiography of balloon angioplasty, bare metal stents, brachytherapy, and drug-eluting stents has repeatedly indicated that the longitudinal topographical location of the MLA may change from its preprocedural site to its post-procedural site and to its follow-up site. The acute relocation (pre procedure/post procedure) is related to acute mechanical treatment, while the late topographical relocation (post procedure/follow-up) is a phenomenon more related to the long-term biological reaction of the vessel wall.
Relocation of the MLA after implantation of a BRS has not yet been studied. Therefore, we studied MLA relocation using IVUS as a post hoc study of the ABSORB II trial, which compared the Absorb™ everolimus-eluting BRS and the XIENCE metallic everolimus-eluting stent (EES) (both Abbott Vascular, Santa Clara, CA, USA) in the context of a randomised trial. The aims of this study were 1) to compare acute and late MLA relocation in BRS with relocation in metallic EES as well as the serial long-term morphometric changes of the initial preprocedural MLA site at three-year follow-up, and 2) to attempt to unravel the main determinants of late relocation phenomenon.
Methods
STUDY DESIGN AND POPULATION
The ABSORB II trial was a prospective, single-blind, multicentre clinical trial that randomised patients to percutaneous coronary intervention (PCI) with either BRS or metallic EES in a 2:1 fashion. The trial design, the study devices, and the inclusion and exclusion criteria have been described in detail previously1. As mandated by the protocol, all patients underwent documentary greyscale IVUS and backscattered radiofrequency assessment before and after device implantation and at three-year follow-up. The ABSORB II trial was funded by Abbott Vascular.
IVUS IMAGE ACQUISITION AND ANALYSIS
IVUS data were acquired with a 3.2 Fr, 45-MHz rotational IVUS catheter (Revolution® 45 MHz; Volcano Corporation, Rancho Cordova, CA, USA) after intracoronary injection of 200 µg of nitroglycerine, at a pullback speed of 0.5 mm/second and a frame speed of 30 frames/second. All pullbacks were analysed off-line at 1 mm longitudinal intervals by an independent core laboratory (Cardialysis BV, Rotterdam, the Netherlands) using commercially available software (QIvus version 2.2; Medis, Leiden, the Netherlands).
DEFINITIONS OF IVUS PARAMETERS
The methods of quantitative IVUS have been published previously2. Vessel contour was delineated along the border between the media and the adventitia. Lumen contour was delineated along the border between flowing blood and the vessel wall. In case of malapposition, the lumen border was assessed behind the struts. In order to compare the two devices, and considering the difficulty of measuring the neointima in the biodegraded scaffold at three years, the intra-scaffold/stent neointima was included in the metric “plaque/media = vessel area – lumen area”. Plaque area increase or “plaque growth” is a metric name used to describe, in a combined fashion, tissue growth behind polymeric or metallic struts, and tissue growth intra-scaffold or intra-stent3,4. The reference segments after device implantation were the 5 mm segments proximal and distal to the device.
Definitions of expected balloon-artery ratio, expansion index, asymmetry index, eccentricity index as well as details of the analysis method in preprocedural IVUS-VH are described in Supplementary Appendix 1.
DEFINITION OF RELOCATION OF MLA
We defined the position of the MLA in the scaffold/stent, either pre procedure, post procedure or at follow-up, as the distance from the distal edge of the scaffold/stent to the MLA site. Previously, Arbab-Zadeh et al reported that the average IVUS probe motion during the cardiac cycle was 2.43±1.42 mm as assessed by cineangiography5. Accordingly, relocation was considered as a present phenomenon whenever longitudinal MLA position changed by more than 2.4 mm, either proximally or distally. Acute relocation was defined as relocation from pre procedure to post procedure; late relocation was defined as relocation from post procedure to follow-up (Figure 1).
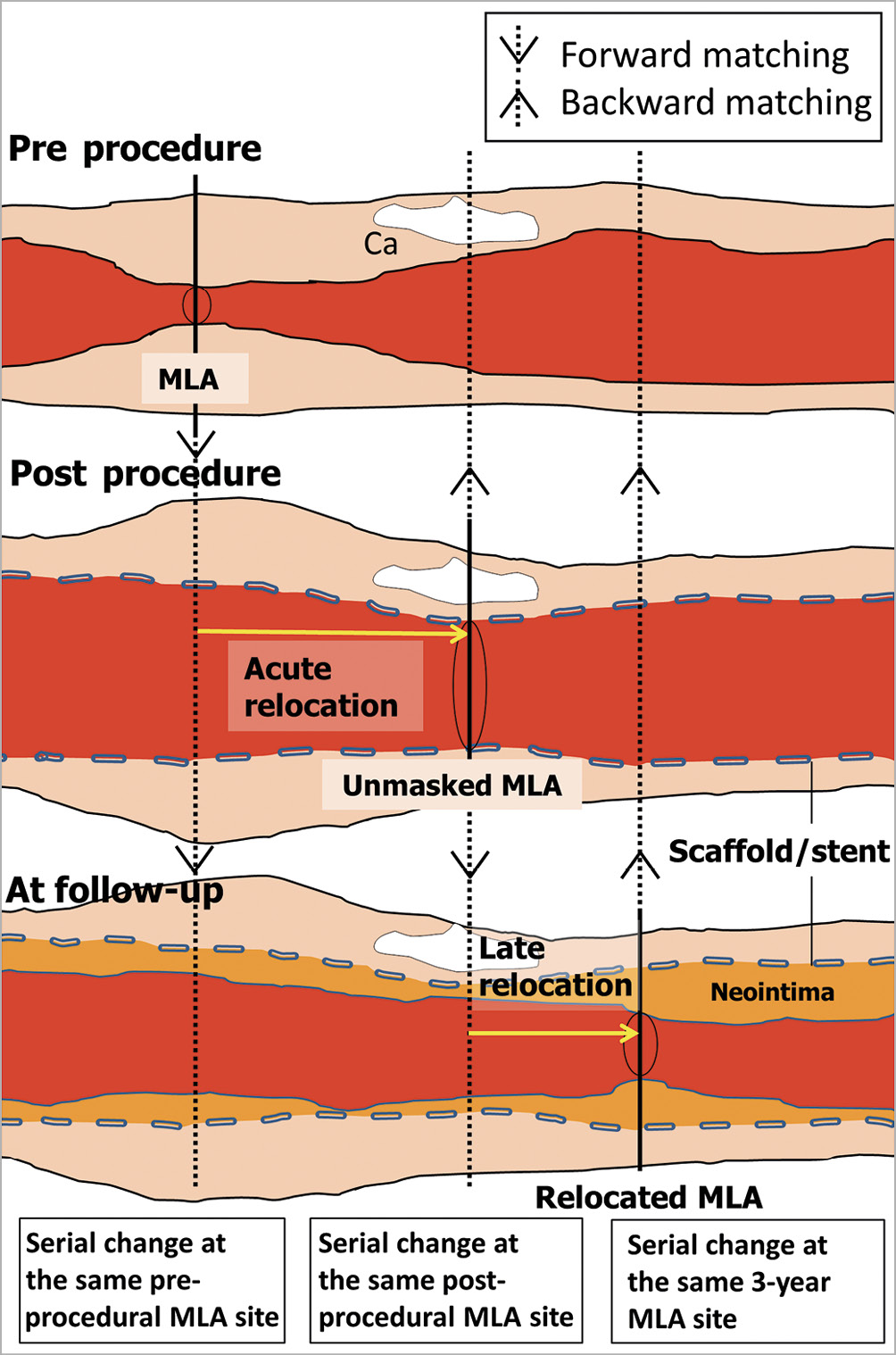
Figure 1. Matching the MLA site on IVUS at various time points. Pre-procedure, post-procedure and follow-up MLA cross-sectional images were colocalised prospectively (↓) and retrospectively (↑) and serially analysed for assessment of lumen and vessel area. Ca: calcification; MLA: minimal lumen area
MATCHING MLA LOCATION WITH IVUS AT DIFFERENT TIME POINTS (PRE PROCEDURE, POST PROCEDURE, FOLLOW-UP)
After identifying the frame of the MLA site, matching with IVUS at a different time point was performed by colocalising common landmarks6 (Figure 1). The preprocedural MLA cross-section was matched with the corresponding cross-section post procedure and at three years. The post-procedural MLA cross-section was matched with the corresponding cross-section pre procedure and at three years. The three-year MLA cross-section was matched with the corresponding cross-section pre procedure and post procedure. In addition, the post-procedural MLA cross-section was matched with the preprocedural corresponding site of IVUS-VH for plaque compositional analysis.
STATISTICAL ANALYSIS
The method of statistical analysis is described in Supplementary Appendix 1.
Results
BASELINE CHARACTERISTICS
In the ABSORB II trial, out of the 501 patients enrolled, a complete set of IVUS pre procedure, post procedure, and at three years was available in 237 lesions (224 patients) in the BRS arm and 129 lesions (120 patients) in the EES arm (Figure 2). Baseline clinical and lesion characteristics in those patients/lesions were well balanced between both arms (Supplementary Table 1). In terms of procedural characteristics, maximal pressure during device implantation or post-dilatation, as well as the nominal diameter of the post-dilatation balloon, were significantly higher in the EES arm than in the BRS arm.
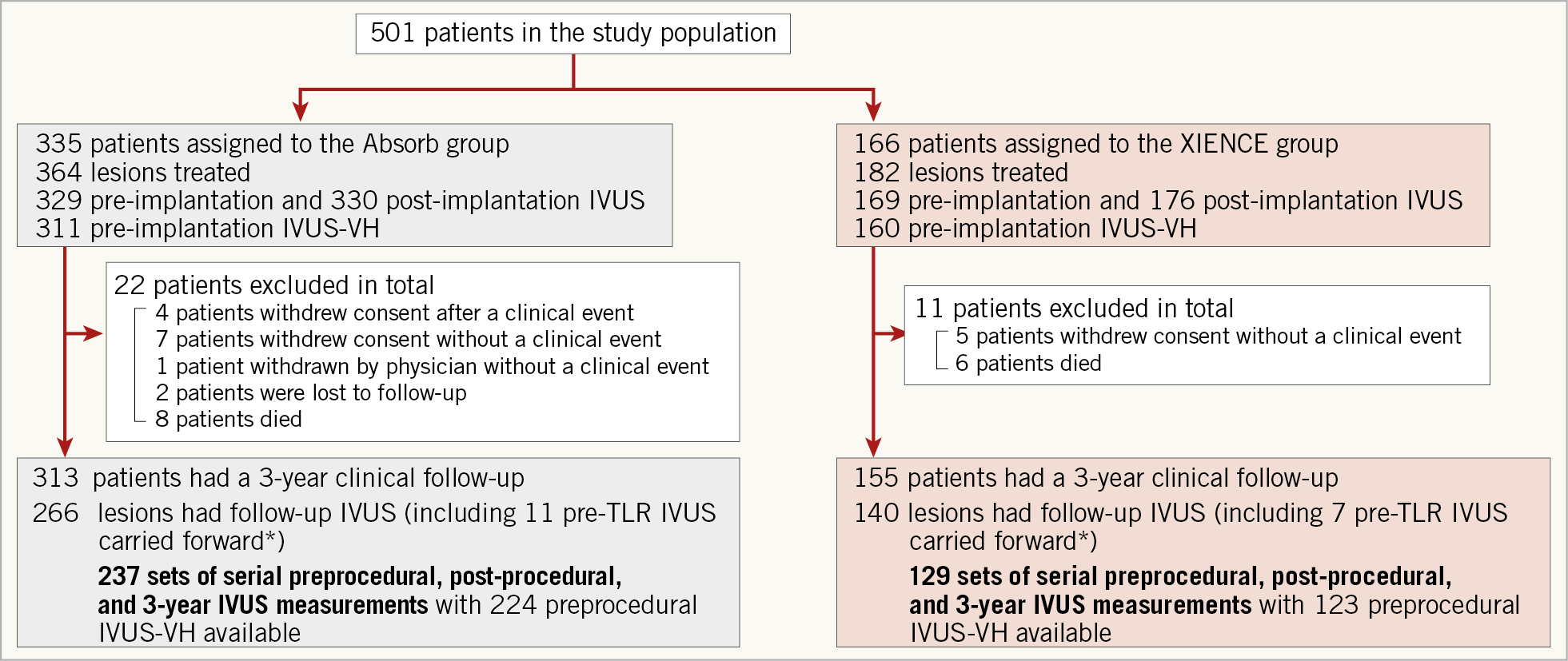
Figure 2. Study flow chart. *In case of TLR, the results of pre-TLR IVUS were carried forward to 3Y for statistical purpose. IVUS: intravascular ultrasound; MLA: minimal lumen area; TLR: target lesion revascularisation; VH: virtual histology
INCIDENCE OF ACUTE AND LATE RELOCATION
In 366 lesions with a complete set of IVUS pre procedure, post procedure, and at three-year follow-up, 166/366 (45.4%) lesions did not have acute relocation (residual MLA) (Figure 3). In these lesions without acute relocation, MLA in 59/166 (35.5%) lesions stayed at the same site at pre procedure, post procedure, and three-year follow-up, while 107/166 (64.5%) lesions showed late relocation at three years.
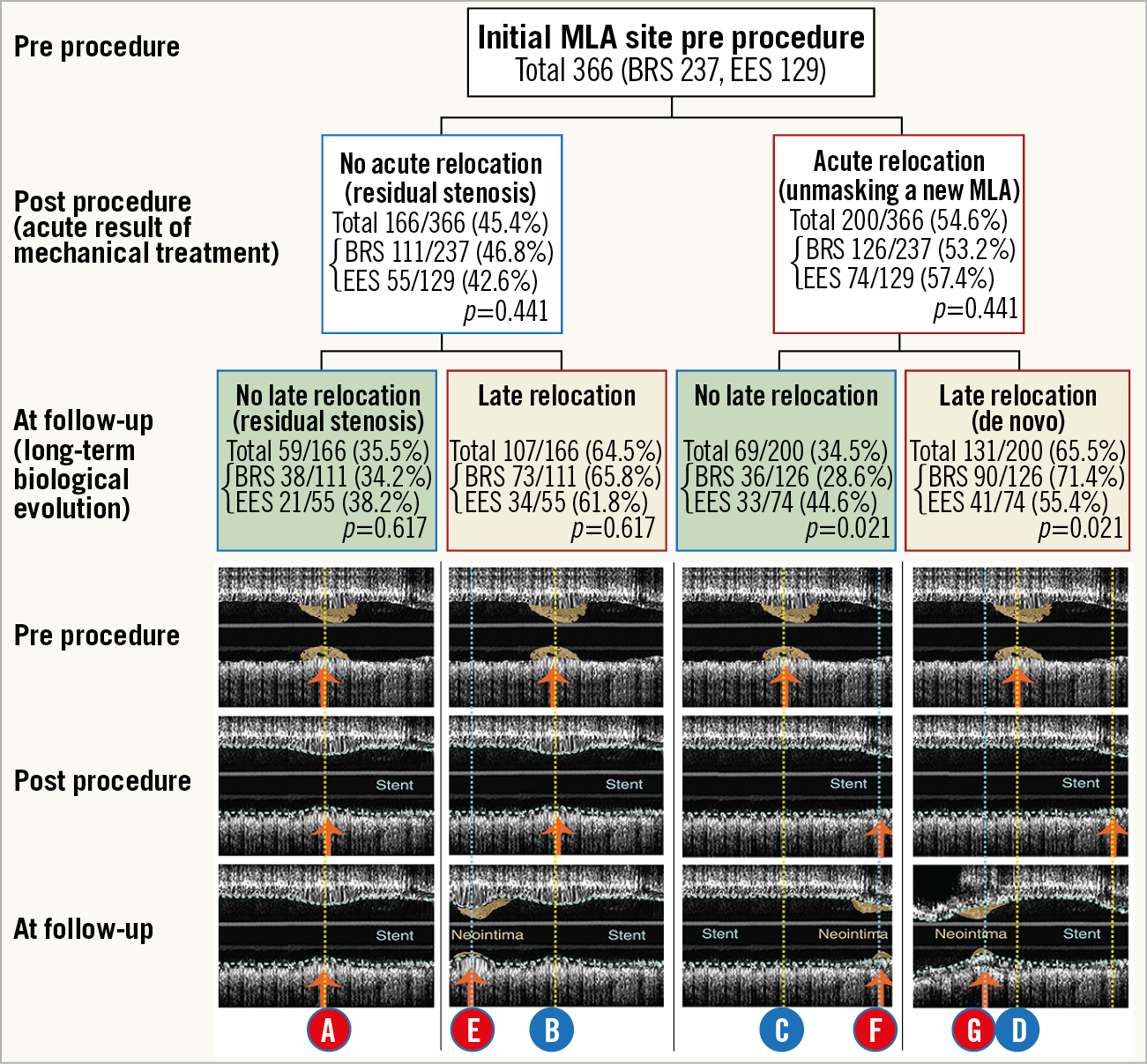
Figure 3. Serial change of longitudinal position of MLA (truly serial IVUS pre procedure, post procedure, and at three-year follow-up: n=366 lesions). Acute or late MLA relocation is defined as either a proximal or distal shift greater than 2.4 mm from pre procedure to post procedure or post procedure to follow-up. Yellow dotted lines indicate a cross-section matched post procedure or at follow-up with the initial site of the preprocedural MLA. Line A: in lesions without any acute or late relocation; line B: in lesions without acute but with late relocation; line C: in lesions with acute but without late relocation; and line D: in lesions with acute and late relocation. Blue dotted line indicates the MLA cross-section at follow-up “back-matched” with pre-procedure or post-procedure cross-sections. Line E: in lesions without acute but with late relocation; line F: in lesions with acute but without late relocation; line G: in lesions with acute and late relocation.
In lesions with acute relocation (200/366), 69/200 (34.5%) so-called “unmasked” MLA lesions remained at three years the MLA at the same site, whereas at follow-up a de novo MLA superseded the “unmasked” post-procedural MLA in 131/200 (65.5%) lesions.
Rates of late relocation in lesions without acute relocation were similar between the two arms (65.8% vs 61.8%, p=0.617), whereas, in lesions with acute relocation, late relocation was significantly more frequent in the BRS arm than in the EES arm (71.4% vs 55.4%, p=0.021) (Figure 3).
Overall, the proportion of lesions with late MLA relocation was greater in the BRS arm than in the EES arm (163/237 [68.8%] vs 75/129 [58.1%], p=0.041) (Supplementary Figure 1).
COLOCALISED CHANGES OF THE INDEX PREPROCEDURAL MLA AT POST PROCEDURE AND AT FOLLOW-UP
Serial changes of lumen, plaque, and vessel area of the index preprocedural MLA site are shown in Figure 4. When all lesions were combined regardless of relocation status, BRS showed significant late lumen enlargement with expansive remodelling overcompensating for significant “plaque growth”, whereas EES showed significant late lumen narrowing with significant expansive remodelling unable to compensate for a significant “plaque growth” at the preprocedural MLA site from post procedure to three-year follow-up (Figure 4A).
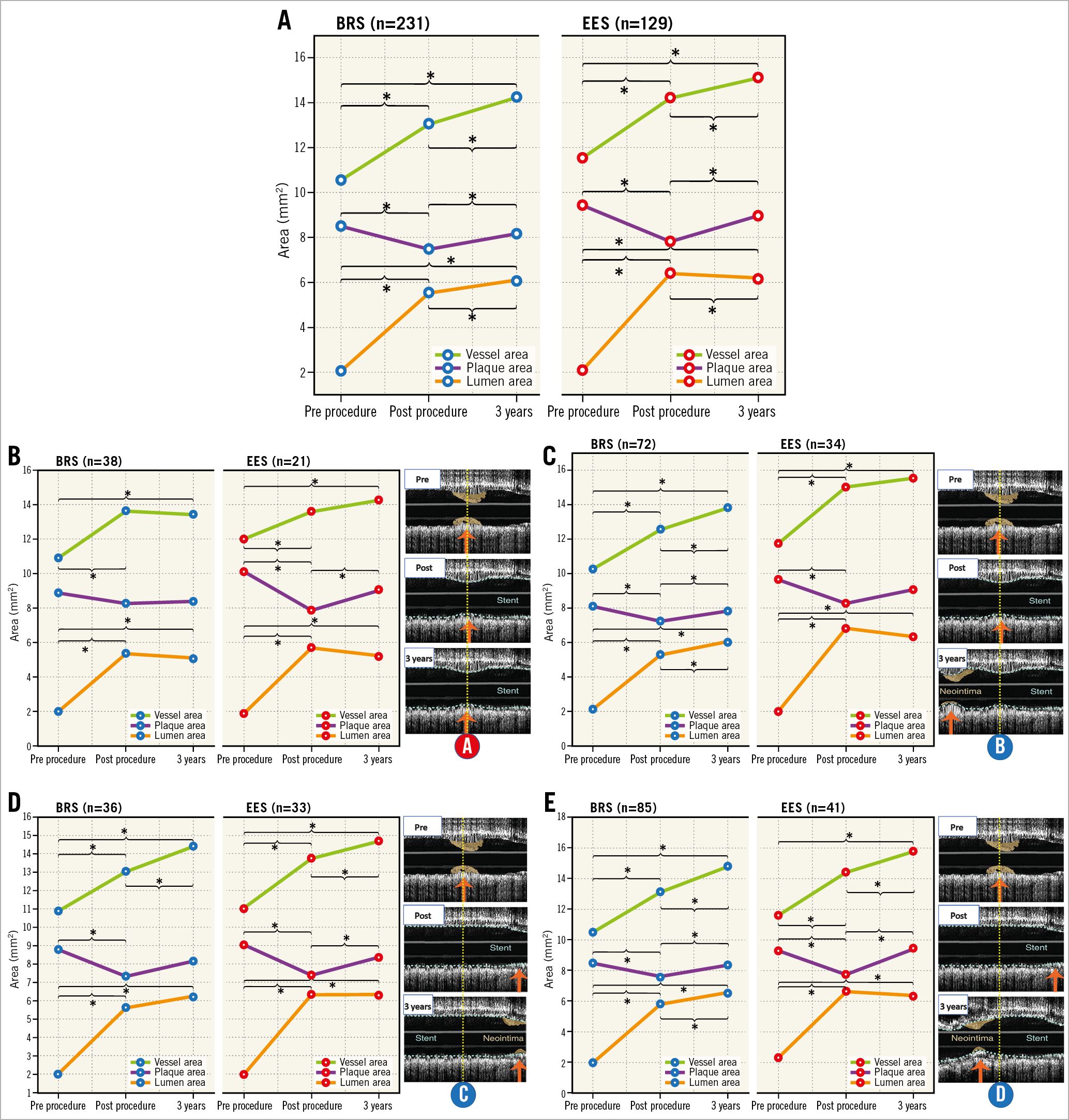
Figure 4. Serial changes (post procedure, three years) of lumen, plaque, and vessel area at the initial site of preprocedural MLA. A) Serial changes at the preprocedural MLA site regardless of relocation status. Aggregated data from lines A, B, C, and D in Figure 3. B) Serial changes at the preprocedural MLA site without any acute or late relocation (the same MLA site pre procedure, post procedure, at three years). Line A in Figure 3. C) At the site of preprocedural MLA in lesions without acute relocation but with late relocation. Line B in Figure 3. D) At the site of preprocedural MLA with acute relocation but without late relocation. Line C in Figure 3. E) At the site of preprocedural MLA with acute and late relocation. Line D in Figure 3. All serial changes except plaque area in BRS in panel B were significant in repeated measures ANOVA. p-values for pairwise comparisons were adjusted by Bonferroni correction. *p<0.05. BRS: bioresorbable scaffold; EES; everolimus-eluting stent
In lesions without relocation (no acute or late relocation), no significant change in lumen area or vessel area was observed in both BRS and EES arms from post procedure to three years (A in Figure 3, Figure 4B).
At the preprocedural MLA site in lesions without acute but with late relocation, BRS showed significant late lumen enlargement with significant expansive remodelling overcompensating for a significant “plaque growth” from post procedure to three years. On the other hand, EES did not show any significant change in lumen, plaque, or vessel area from post procedure to three years (B in Figure 3, Figure 4C).
Initial preprocedural MLA, in lesions with acute MLA relocation but without late relocation, showed a non-significant increase in lumen and plaque area with significant expansive remodelling in BRS; EES showed stable lumen area with significant “plaque growth” compensated for by significant expansive remodelling from post procedure to three years (C in Figure 3, Figure 4D).
In lesions with both acute and late relocation, BRS showed significant late lumen enlargement with expansive remodelling overcompensating for a significant “plaque growth”; EES showed no significant change in lumen area with significant “plaque growth” compensated for by expansive remodelling from post procedure to three years (D in Figure 3, Figure 4E).
BACKWARD MATCHING PRE AND POST PROCEDURE OF THE THREE-YEAR MLA
When lesions with any acute or late MLA relocation (E, F, and G in Figure 3) were combined, both BRS and EES showed significant lumen reduction due to plaque increase and constrictive remodelling at the three-year MLA site from post procedure to three years (Supplementary Figure 2). The analyses in subgroups are included in Supplementary Appendix 2.
MULTIVARIATE ANALYSIS FOR PREDICTORS OF LATE MLA RELOCATION
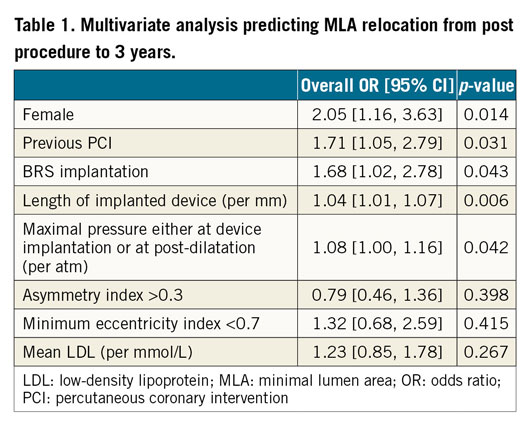
Univariate analyses for predicting late MLA relocation at three years were performed, with the result tabulated in Supplementary Table 2. Subsequent multivariate analysis retained the following in the model as independent predictors of late MLA relocation: female gender, previous PCI, BRS implantation, total device length, and maximal pressure either at device implantation or at post-dilatation (Table 1).
Discussion
MAIN FINDINGS
The main findings of this study are the following. 1) Late MLA relocation was more frequent in lesions treated with BRS than in those treated with EES. 2) At the original site of MLA, late lumen enlargement with expansive remodelling overcompensating for “plaque growth” occurred in the BRS arm, whereas in the EES arm significant lumen reduction due to significant plaque growth not compensated for by expansive remodelling was observed over three years. 3) The relocated three-year MLA site was characterised by lumen area reduction due to plaque progression in combination with constrictive remodelling in both arms. 4) Female gender, previous PCI, BRS implantation, total device length, and maximal pressure during the procedure were independent predictors of late MLA relocation.
The current study is not limited to a description of MLA relocation, but also shows the physiopathological impact of pressure applied to the luminal wall in the treated segment. On the one hand, high pressure during the procedure may promote strut embedment and expansive remodelling, while it can promote neointimal hyperplasia by inflammatory reaction7,8. On the other hand, low pressure may result in poor embedment and excessive strut protrusion into the lumen and causes an area of low shear stress resulting in an early phase, fibrin deposition, thrombosis and exuberant neointimal hyperplasia and in a late phase neoatherosclerosis9. In a previous report by Stone et al10, optimal predilatation (with a balloon to QCA-RVD ratio ≥1:1) was associated with greater one-year rates of target lesion failure (TLF), while optimal post-dilation (with a non-compliant balloon at ≥18 atm and with a nominal diameter larger than the nominal scaffold diameter, but not >0.5 mm larger) was associated with lower TLF rates between one and three years after BRS implantation. In their report, the pathophysiological reason why “optimal predilatation” and “optimal post-dilatation” work oppositely was unclear. Importantly, in the present study, we identified maximal pressure during the procedure as an independent determinant of late MLA relocation, suggesting its physiological impact on de novo stenosis at follow-up. However, we could not find statistical significance in relationships between MLA relocation and clinical outcomes due to the limited number of events (Supplementary Table 3).
COMPARISON WITH PREVIOUS LITERATURE
Sabaté et al reported that late MLD relocation was observed in 78.5% of lesions treated with brachytherapy and only in 26.3% of lesions treated with balloon angioplasty (p<0.0001)11. Costa et al reported that MLD relocation on angiography occurred in 42.8% with BMS and 33.7-36.4% with DES at nine-month follow-up12. Our results show a rate of late MLA relocation on IVUS of 59.6% with metallic EES, which is higher than that reported by Costa et al. The longer follow-up period of our population (three years vs nine months) might have resulted in a different impact of neointimal growth on MLA relocation. On the other hand, the rate of MLA relocation with BRS in the present study was higher than in lesions treated with balloon angioplasty in the work previously reported by Sabaté et al.
The Absorb bioresorbable scaffold is considered to lose its mechanical support at six to 12 months, with a complete polymer resorption at three to four years13. However, future research is needed to understand how the scaffold influences the change in lumen configuration between loss of mechanical support and complete absorption.
PREDICTORS OF LATE MLA RELOCATION
BRS implantation, female gender, balloon-artery ratio, expansion index, previous history of PCI, and low-density lipoprotein (LDL) were independent determinants of expansive remodelling in our previous report based on device-level analysis14. Most of these predictors of device-level expansive remodelling overlap with the predictors of late MLA relocation in the present cross-sectional level analysis. Indeed, univariate logistic regression analysis shows that expansive remodelling (defined as mean vessel area increase >12%) has an odds ratio of 1.98 (95% confidence interval: 1.14-3.44, p=0.015) predicting late MLA relocation. Although we could not include expansive remodelling (yes/no) in the multivariate model considering multicollinearity with other factors, it is quite possible that expansive remodelling is related to late MLA relocation.
Female gender appears to be an independent predictor of late MLA relocation. Previously, Trabattoni et al reported that female gender was more frequently associated with in-stent restenosis as compared to male gender15. Moreover, females had more diffuse in-stent restenosis (ISR) (71.8%) than males (40.3%). In lesions with diffuse restenosis, MLA relocation may occur more easily.
In the present study, previous PCI (early onset of coronary artery disease) was an independent predictor of late MLA relocation. Although prior PCI has been identified as a predictor of target lesion revascularisation16, the impact on MLA relocation has not been reported.
Total device length is associated with late MLA relocation. As the device length increases, it is reasonable to postulate that a topographical restenotic process in a locus other than the original MLA will be more likely.
After adjusting for other patient characteristics and procedural factors, BRS implantation is still an independent predictor of late MLA relocation. The vessel treated with BRS is free from caging when the mechanical integrity of the scaffold is lost. It has been shown that late loss is higher in BRS than in EES1, whereas lesions treated with BRS have more potential for vessel remodelling as compared to metallic stents14. However, the site of remodelling or lumen reduction may be difficult to explain solely by the factors discussed above.
Previous literature has shown that higher pressure may trigger both neointimal hyperplasia and vessel remodelling7,8. In the current study, maximal pressure during the procedure was an independent determinant of late MLA relocation. Serial and matched change of initial preprocedural MLA site showed late lumen enlargement with expansive remodelling in BRS, while late lumen reduction was observed in EES so that lumen area at follow-up was almost identical (6 mm2) in both arms (Figure 4A). In case of acute or late relocation, both arms exhibited late lumen reduction due to constrictive remodelling and “plaque growth” (Supplementary Figure 2A). High pressure at the site of the index MLA has a beneficial effect in the BRS arm long term, while high pressure may trigger in both arms relocation that is associated with long-term deterioration of the lumen at sites other than the index stenotic site. In other words, high-pressure implantation or post-dilatation might be viewed as a double-edged sword10. These observations are hypothesis-generating but would suggest either the use of highly pressurised non-compliant balloon delivery with an oversized mid part of the balloon or the use for post-dilatation of a short, oversized balloon centred on the minimal lumen area (both not >0.5 mm larger than the nominal diameter of the scaffold to avoid fractures in the BRS) (Figure 5).
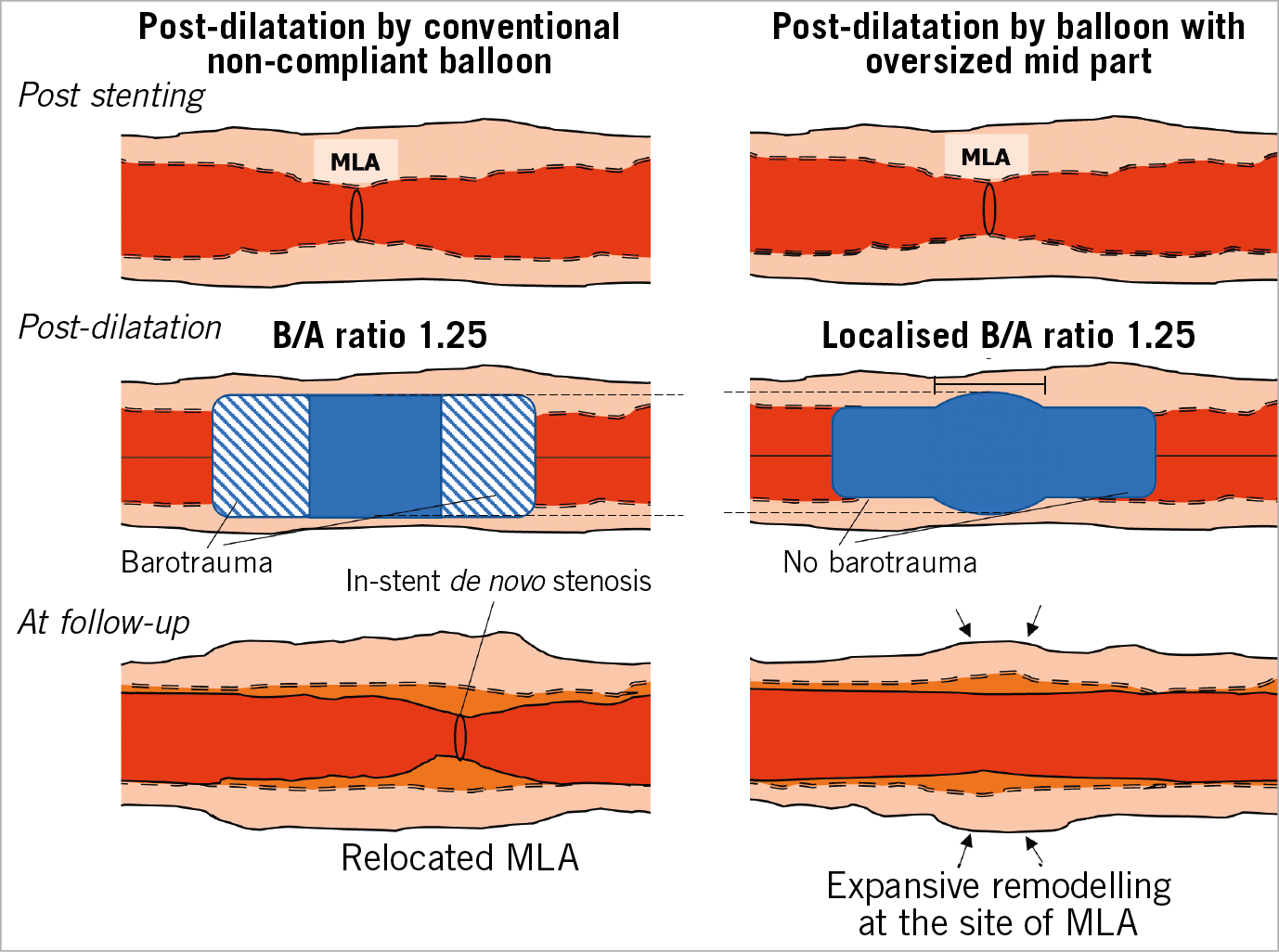
Figure 5. Difference between pressure applied along the balloon length and to a localised region. Maximal pressure either during device implantation or at the time of post-dilatation is one of the independent determinants of late MLA relocation as well as BRS implantation. High pressure at the site of the index MLA has a beneficial effect, while at sites other than the index stenotic site high pressure may trigger MLA relocation that is associated with long-term deterioration of the lumen. The observation suggests either the use of highly pressurised non-compliant balloon delivery with an oversized mid part of the balloon or the use for post-dilatation of a short, oversized balloon centred on the minimal lumen area. B/A ratio: balloon-artery ratio; MLA: minimal lumen area
Although none of the geometric parameters (i.e., asymmetry index or eccentricity index) was identified as an independent factor predicting late MLA relocation in this study, luminal configuration affects shear stress, which is considered to impact on neointimal formation9. To understand the underlying mechanism of late MLA relocation fully, the influence of shear stress should be taken into account and investigated.
Study limitations
Matching analysis inherently suffered from longitudinal error due to cardiac motion. In a study in saphenous vein grafts after stent implantation, QCA-derived MLD position and IVUS-derived MLD failed to correlate17, which may be accounted for by the longitudinal inaccuracy of QCA due to vessel foreshortening, whereas cross-sectional and longitudinal IVUS measurement is not affected by 2D geometric limitation. Optical coherence tomography (OCT) was not available in ABSORB II. Although OCT has better longitudinal accuracy with faster pullback speed, it still suffers from longitudinal error18. Due to bioresorption of the BRS up to three years, the core lab could not measure the scaffold area at three years in 3,737 frames among 6,531 frames analysed in the BRS arm. Therefore, a separate analysis of neointima and plaque behind struts could not be performed and the analysis focused on total plaque area.
Conclusions
In the ABSORB II trial, late MLA relocation was more frequent in lesions treated with BRS than in those treated with EES. Late lumen enlargement and expansive vessel remodelling at the site of the initial preprocedural MLA was observed in BRS, but not in EES. At the same time, high pressure may trigger relocation associated with long-term lumen narrowing and constrictive remodelling at sites other than the index stenotic site. The implantation technique and the future design of balloon technology may impact on the long-term outcome of stenting and scaffolding but warrant future prospective investigation.
|
Impact on daily practice Coronary lesions treated with BRS more frequently exhibit MLA relocation from post procedure to follow-up than those treated with EES. Late lumen enlargement and expansive vessel remodelling at the site of initial preprocedural MLA was observed in BRS but not in EES. Considering that maximal pressure during the procedure is one of the identified independent predictors of MLA relocation from post procedure to follow-up, high pressure might not be applicable consistently along the device segment but should be considered to be applied locally. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
This study was funded by Abbott Vascular.
Conflict of interest statement
P.W. Serruys is a member of the Advisory Board for Abbott Vascular. B. Chevalier is a consultant for Abbott Vascular. Y. Onuma is a member of the Advisory Board for Abbott Vascular and has received speaker honoraria from Terumo. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

