Abstract
Aims: Preclinical studies and translational animal models are fundamental for the development of new clinical interventions. Compared to human anatomy, pigs present a more anterior heart position in the chest which may jeopardise the imaging and testing of devices designed to be delivered to the human mitral valve. To imitate human anatomy, we developed a novel model to “humanise” a pig heart.
Methods and results: The creation of a neo inferior vena cava with a Dacron tube grafted to the right atrium was tested for transseptal delivery of an experimental mitral annuloplasty device in 35 animals. In 15 animals with native anatomy a conventional right transfemoral access was used. Imaging guidance was achieved with intracardiac or epicardial echocardiography. In all transfemoral approaches (n=15), the delivery of the device was unsuccessful and the handling was dissimilar to a human implant. In all neo-cava approaches (n=35), the handling and manoeuvring were as expected in humans, the targets were reached as intended and all procedures but one were successful.
Conclusions: A translational “humanised” animal model with the creation of a neo cava eliminates the differences between pig and human anatomy and is suitable for testing human grade devices.
Introduction
Reliable translational animal models are necessary to demonstrate proof-of-concept of new interventions and devices. Most preclinical studies are based on either sheep or pig models1,2. Despite the similarities with human anatomy, pigs have a more anterior heart position in the chest compared to humans3, resulting in challenging imaging and device delivery (Figure 1). To overcome the dissimilarities, we developed a model that “humanises” porcine anatomy: the inferior vena cava is relocated creating the same angle to the mitral annulus as seen in humans to facilitate deliverability of mitral valve devices.
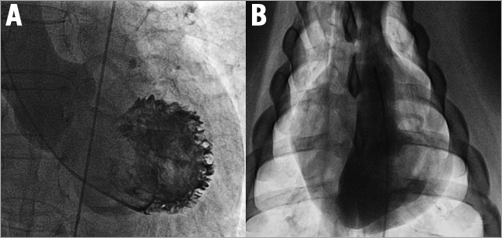
Figure 1. Different orientation of human and pig left ventricle. A) Anteroposterior view: long axis of the human heart pointing to the left, caudal. B) Anteroposterior view: long axis of the pig heart pointing to the right, caudal.
Methods
The animal studies were conducted in two facilities in compliance with Italian Ministry of Health national law (116/92), European Union guideline (86/609/EEC) and Swiss Federal animal protection law and ordinance (licence 138/2010 issued by Zürich Cantonal Veterinary Office).
Fifty female farm pigs were used and prepared using standardised protocols. Monitoring known from human cardiac surgery was applied. All animals underwent the same interventional procedure (endovascular implantation of an annuloplasty ring intended to treat mitral regurgitation: Cardioband; Valtech Cardio, Or Yehuda, Israel), but were divided into two groups with different access: group one (n=15) with right transfemoral/transseptal approach (conventional) and group two (n=35) with the novel approach through a right lateral thoracotomy creating a neo cava with a 10 mm Dacron tube grafted to the right atrium (Figure 2). The graft was connected to a custom-made haemostatic valve and oriented with a 45° angle to the midline of the animal to reproduce a “human-like” angulation between the vena cava and the mitral annulus (Figure 3).
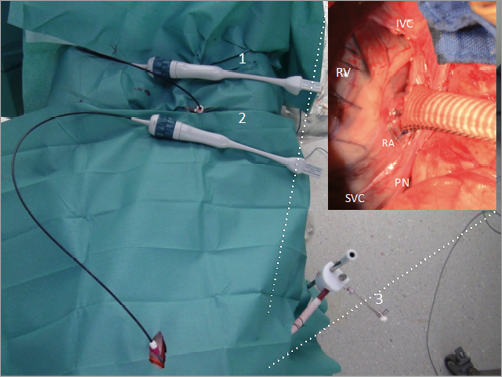
Figure 2. Set-up. 1) Intracardiac echo probe through right femoral vein. 2) Intracardiac echo probe through apical approach. 3) Neo-cava graft with approximately 45° orientation. The inset picture shows the neo-cava graft. IVC: inferior vena cava; PN: phrenic nerve; RA: right atrium; RV: right ventricle; SVC: superior vena cava
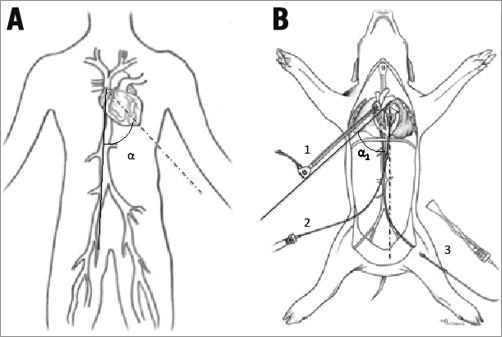
Figure 3. The different anatomy of human and pig hearts. A) Human: the angle (α) between the axis of the IVC (continuous line) and the long axis of the heart (dotted line) is greater than 45°. B) Pig: the axis of the native IVC and the long axis of the heart (dotted line) are parallel. With the Dacron neo cava (continuous line), the new angle (α1) is similar to humans. 1: neo cava; 2: transapical ICE; 3: transfemoral ICE
To overcome the limitation of transoesophageal echocardiography due to minimal contact of the pig oesophagus with the left atrium, we used intracardiac echocardiography (ICE). To provide concomitant short- and long-axis views of the annulus, we modified the standard approach4 and used two probes instead of one (Figure 3): femoral vein for transseptal puncture and mitral short-axis view (Figure 4A, Figure 4D), and transapical access for detailed posterior annulus long-axis view (Figure 4B, Figure 4C).
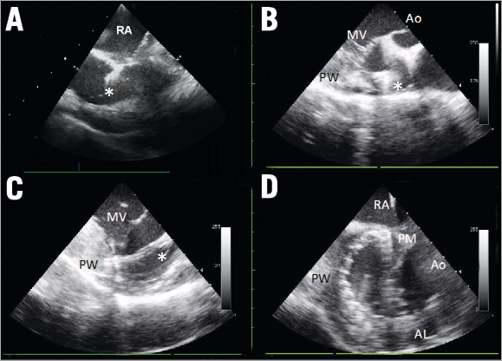
Figure 4. Intracardiac echo. A) Short axis from transfemoral to guide transseptal puncture: fossa ovalis is tented by the needle (asterisk). B) Long axis from transapical: aortic valve (Ao), mitral valve (MV), left ventricle posterior wall (PW). Asterisk showing the implant pointing towards the target in the anterolateral commissure. C) Long axis from transapical: mitral leaflets (MV). Asterisk indicates the implant connected to the target in the annulus. D) Short axis from transfemoral: implant situated on the entire posterior annulus from the posteromedial commissure (PM) to the anterolateral commissure (AL). RA: right atrium
Due to the different orientation of the heart relative to the chest cavity in the animal model (Figure 5A), a caudal anteroposterior projection (Figure 5B, Figure 5C) corresponds to left anterior oblique projection in humans (Figure 5D). The aim of the study was to analyse deliverability of the implant. Success was defined as full deployment along the posterior annulus. Unsuccessful implant was defined as a gap >1 cm between the implant and the commissures.
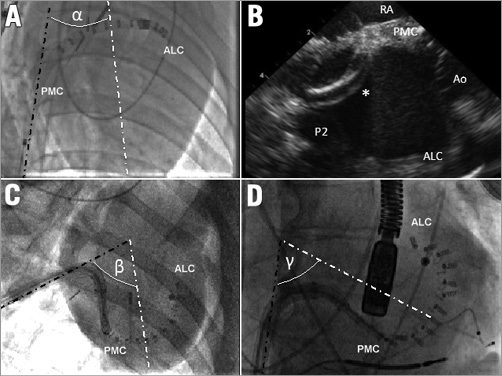
Figure 5. Effect of neo cava on device delivery. A) Conventional animal approach: the angle (α) between the IVC (dark dotted line) and the left ventricle (white dotted line) is very narrow. B) Although the delivery system (asterisk) is fully steered, its tip does not reach the posteromedial commissure (PMC), and barely touches the posterior annulus (P2). C) & D) Delivering the final anchors of the implant into the PMC: anteroposterior caudal view in the neo-cava approach (C), and left anterior oblique view in a human using the same device (D). With the neo-cava approach (C), the IVC (dark dotted line) and the left ventricular long axis (white dotted line) angle (β) is similar to the angle (γ) observed in humans. The curves on the delivery system at the same stage of implant are comparable in (C) and (D), demonstrating the reproducibility of the animal delivery in humans. ALC: anterolateral commissure; Ao: aortic valve; RA: right atrium
Results
Procedural timing is shown in Table 1. Operators assessed ease-of-use of the delivery system with a subjective usability score (Table 2). In the conventional approach, the manoeuvrability of the device was unlike in humans (tight angle between native vena cava and the heart) and the implant was never successful due to the limited steering of the system to target the posteromedial annulus (Figure 6A, Figure 6B). Through the neo-cava approach, the delivery was successful in all but one animal because the handling and manoeuvring were as in humans. All targets were reached without need for excessive steering (Figure 6C, Figure 6D). The neo-cava approach (Figure 5C) increased the angle between the inferior vena cava and the left heart long axis (Figure 5B), reproducing human anatomy (Figure 5D).
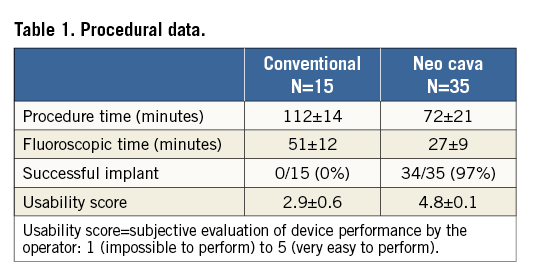
Figure 6. Post-mortem study following annuloplasty in conventional (A & B) vs. neo-cava animals (C & D). A) Simple anchoring (without device) into the annulus with anchors located distally to the commissures. B) Unsuccessful implant with asterisk indicating the last anchor. The anchors are far away from the commissures. C) & D) Successful implants: anchors located correctly at the level of the commissures.
Discussion
Our study shows that a translational “humanised” animal model for transcatheter mitral therapies enables improved deliverability of an annuloplasty device using a “human grade” delivery system. It eliminates the differences between human and animal anatomy resulting in near 100% successful implants while using systems designed for humans in an animal model that would otherwise result in unsuccessful delivery (conventional group) due to technical difficulties caused by different animal anatomy. It also allows for a device insertion in a straight line, as opposed to the tortuous access of the animal femoral veins in the conventional approach.
When the “human grade” device was delivered by standard transfemoral access in the animal model, the implant was never successful because the posteromedial commissure was not reachable. The handling of the device was neither predictable nor comparable to standard human manoeuvring for other mitral delivery systems.
The limitations of this model are the need for a surgical vascular access requiring a multidisciplinary experimental “Heart Team”, and the fact that the two groups were used in sequence. Therefore, the effect of the learning curve cannot be excluded.
In conclusion, although heart anatomy is very similar among mammals, the great vessel anatomy and heart orientation differ between humans and quadrupeds. Such differences may jeopardise animal testing of devices designed to be delivered in humans. A “humanised” animal model involving the creation of a neo inferior vena cava to reproduce the same angle of approach to the heart as in humans is an efficient method to test deliverability of “human grade” devices for mitral valve transcatheter interventions. In addition, multiple ICE catheters can provide ideal imaging to guide experimental interventions.
| Impact on daily practice Preclinical translational animal models are fundamental for the development of new clinical interventions. Compared to human anatomy, pigs have a different heart position which may jeopardise testing of devices designed for human hearts. To imitate human anatomy, we developed a novel model to “humanise” a pig heart: we created a neo inferior vena cava with a Dacron tube grafted to the right atrium for transseptal delivery of an experimental mitral annuloplasty device. In all neo-cava animals (n=35) the handling and manoeuvring was as expected in humans, the targets were reached and all procedures but one were successful. Our “humanised” animal model with a neo cava eliminates the differences between pig and human anatomy and is suitable to test human grade devices. |
Conflict of interest statement
F. Maisano, V. Falk, P. Denti, A. Addis and A. Guidotti are consultants for or have received research grants from Valtech Cardio or Abbott Vascular. The other authors have no conflicts of interest to declare.

