Abstract
Aims: Perlecan is the major heparan sulfate proteoglycan in the arterial wall. Previous studies have suggested that perlecan is a potent inhibitor of smooth muscle cell (SMC) activity. Therefore, perlecan over-expression may serve as a therapeutic modality to prevent in-stent restenosis (ISR). We have investigated a novel compound (RUS3108), identified in a SMC based screen to induce perlecan synthesis in SMC. The aims of this study were to assess the in vitro effects of RUS3108 and the effects of RUS3108-eluting stents in preventing ISR.
Methods and results: Rabbit aortic SMC and bovine aortic endothelial cells (EC) were used in this study. Immunohistochemistry showed that RUS3108-treated SMC over-expressed perlecan indicating the drug effects. Furthermore, RUS3108 induced a SMC differentiated phenotype by SMembryonic staining. RUS3108 (1 µM) inhibited 3H-thymidine incorporation by >50%, which was completely reversed by a perlecan antibody. RUS3108 also inhibited SMC migration (Boyden chamber) and MMP-9 activity. In contrast, RUS3108 (100nM) modestly stimulated EC 3H-thymidine incorporation by 22% (p<0.02). In vivo, a total of 30 stents were deployed in rabbit iliac arteries as follows: 1) bare metal stents (n=10), 2) polymer only-coated stents (n=10), and 3) polymer-coated stents containing RUS3108 (n=10). Rabbits were sacrificed at four weeks and stented segments were subjected to morphometric analysis. Intimal cross sectional area was significantly lower in the RUS3108-eluting stent group (0.31±0.27 mm2 versus 1.0±0.31 and 1.25±0.51 in the bare metal stents and polymer only coated stents groups, respectively, p<0.0001).
Conclusions: RUS3108 is a novel perlecan-inducing compound, which is a potent inhibitor of SMC activity and a modest stimulator of EC proliferation. RUS3108-eluting stents may serve as an excellent modality for the prevention of ISR.
Introduction
Perlecan is a large (467-kDA) heparan sulfate proteoglycan (HSPG) that is expressed in most extra-cellular matrices (ECM) and basement membranes1,2. It is the major extra-cellular HPSG in the blood vessel ECM. Its core protein consists of five distinct domains with homologies to molecules involved in cell proliferation, lipoprotein uptake and cell adhesion. Each of these domains has exhibited one or more binding sites for a number of ligands including basement membrane components3-6, cell adhesion molecules7, and growth factors8,9.
The biological effects of perlecan are diverse2. Perlecan has been identified in the atherosclerotic intimal lesions of apoE and LDL receptor deficient mice10. Perlecan has been shown to induce high affinity binding of fibroblast growth factor-2 (FGF-2) both to cells deficient in HSPG and to soluble FGF receptors9, thereby promoting angiogenesis11. Perlecan also appears to inhibit in vitro smooth muscle cell (SMC) proliferation12,13, adhesion14, and migration15. In vivo, perlecan has been shown to inhibit thrombosis and endothelial cell-mediated intimal hyperplasia after arterial injury16.
In vivo studies of perlecan administration have been hampered by lack of availability of purified or partially purified perlecan. We have recently synthesised a number of small pyrimidine compounds (MW 326.7) that induce perlecan synthesis in SMC, which were identified in a SMC based screen. The objectives of the present study were to explore the biological effects of RUS3108, a novel perlecan-inducing compound on both vascular SMC and EC and to assess the in vivo efficacy of RUS3108-eluting stents in preventing neo-intimal hyperplasia. Preliminary studies have shown that RUS3108 inhibits SMC proliferation through inhibition of FAK and PTEN signalling proteins that were reported to act intracellular downstream of perlecan (unpublished data).
Methods
In vitro studies
IDENTIFICATION OF PERLECAN-INDUCING COMPOUNDS
These compounds were identified by random screening of combinatorial compound libraries in a smooth muscle based assay for perlecan induction and anti-proliferative activity. RUS3108 was identified through chemical structure activity relationship of hits identified in the random screen. In more details, subconfluent SMCs were serum starved for 24 hours. Cells were then treated with RUS2184 at 5 µM for another 24 hour. Cells were collected and total RNA isolated. 1 ng of total RNA was used for real time PCR. Real time PCR was performed according to the Stratagene protocol for Brilliant SYBR Green QRT-PCR on step assay. Perlecan transcript was normalised by actin; Real time PCR data showed perlecan gene expression is increased by 180% in SMCs when treated with compound at 5 µM for 24 hrs. These compounds were found to have no effect on any other ECM proteins in an In-Cyte cDNA array containing ≈8600 genes.
IMMUNOHISTOCHEMISTRY
Rabbit aortic SMC (103/chamber) were seeded onto 4-chamber culture slides (Costar) and grown to about 60% confluency. Cells were then serum starved for 24 hours and RUS3108 (1 µM) was added in the presence or absence of 10% FCS. Serum-free medium was added to control cells.
For perlecan immunostaining, cells were then fixed at 4ºC using ice-cold methanol for 10 minutes and nonspecific binding was blocked with 3% normal goat serum for 30 minutes. Mouse monoclonal primary antibodies directed against perlecan (1:300 dilution, Zymed, San Francisco, CA, USA) were added and incubated at 37ºC for one hour. Goat anti-mouse cy3-labeled secondary antibody was then added.
The differentiation status of arterial SMC was determined by immunofluorescence staining for smooth muscle myosin heavy chain embryonic (SMemb) cytoplasmic protein to identify the dedifferentiated “synthetic” phenotypes. Cells were fixed using 4% paraformaldehyde for 15 minutes and nonspecific binding was blocked with 1% BSA and 5% normal goat serum for 30 minutes. Mouse monoclonal primary antibodies directed against SMemb (1:3000 dilution, Seikagaku America, Cape Cod, MA, USA) were added and incubated at 4ºC overnight. Goat anti-mouse cy3-labeled secondary antibody was then added followed by incubation with smooth muscle α-actin (1:250 dilution, FITC-conjugated, Sigma-Aldrich Corp, St. Louis, MO, USA). All slides were counterstained with 4’6-diamidino-2-phenylindole (DAPI) (1 µg/ml).
DNA SYNTHESIS
Rabbit aortic SMC and bovine EC from passages 5 and 6 were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 2% penicillin-streptomycin and 10% foetal calf serum. For DNA synthesis, cells were initially seeded at 2 x 104 per well into 24-well plates. SMC and EC were serum starved for 48 hours and then DMEM containing 10% FCS was added in the absence and presence of increasing concentrations of RUS3108. 1.0 µCi/ml of 3H-thymidine (Amersham) was added for 24 hours and DNA synthesis was measured as previously described17.
In order to isolate the effects of perlecan, DNA synthesis assay was repeated for SMC in the presence or absence of 10 µg/ml of anti-perlecan antibody or polyclonal anti-mouse antibody (IgG). All experiments were performed in triplicates.
MIGRATION ASSAY
Confluent SMCs were incubated for 48 hours in 10% FCS and with increasing doses of RUS3108. Cells were trypsinised and re-suspended in serum-free medium containing RUS3108 for 30 minutes at 37°C. Then, cells were added to the upper chamber of a microchemotaxis chambers (Costar, Corning Inc., Corning, NY, USA) with PVP-free polycarbonate filter (8.0 µm pore size) and incubated for five hours at 37°C. The bottom well of the Boyden chamber was filled with 250 µl of serum-free medium containing PDGF-BB (10 ng/ml). Cells that migrated to the lower side of the filters were fixed and stained with the Diff-Quick staining kit (VWR Laboratory). The filters were mounted on glass slides and counted by light microscopy using x100 magnification. The results were expressed as number of migrated cells per high power field (HPF).
GELATINASE ACTIVITY
SMC were grown to confluency in 96 well plates and RUS3108 (1 uM) was added in 100 ul of medium containing 5% FCS in the presence or absence of a control murine IgG antibody or anti-perlecan antibody and incubated for 72 hours. Media was collected, mixed with 4x sample buffer and analysed by gelatin zymography as previously described18. Similarly, confluent EC were incubated in DMEM containing 5% FCS and increasing concentrations of RUS3108 for four days. Media was collected, mixed with 4x sample buffer and electrophoresed on a 10% SDS-polyacrylamide gel containing 0.1% gelatin. Gels were stained with 0.5% Coomassie brilliant blue. Areas of clearing indicated gelatinase activity. In order to isolate the effects of perlecan, MMP zymography was repeated for SMC in the presence or absence of 10 µg/ml of anti-perlecan antibody or polyclonal anti-mouse antibody (IgG).
In vivo
PREPARATION OF RUS3108-COATED STENTS
Stents (3.0 x 18 mm) were provided by Guidant (Santa Clara, CA, USA). The copolymer, poly(ethylene-c-vinyl acetate), was supplied by ARC Pharmaceuticals Inc (Vancouver, BC, Canada). An average of 4.2 mg of RUS-3108 (range 3.3-5.0 mg) was loaded onto the stents.
ANIMAL MODEL
The animal experiments were performed in accordance with guidelines set out by the University of Toronto and approved by the St. Michael´s Hospital Animal Care Committee. Fifteen male New Zealand white rabbits weighing 3.6 to 3.8 kg were fed a regular diet and pre-treated with daily aspirin (81 mg). A total of 30 stents (3.0 x 18 mm [Guidant, Santa Clara, CA, USA]) were deployed in the left and the right iliac arteries. Briefly, rabbits were anesthetised by intramuscular ketamine and xylazine and maintained with isoflurane. The right carotid artery was cannulated with a 5 Fr sheath and a standard angioplasty guide-wire advanced into the right or left iliac artery. Stents were deployed at the same segment of the iliac artery after inflation up to 10 atmospheres for 45 seconds. Stenting using similar inflation pressures and timing was used by a single experienced operator to cause similar vessel injury. Injury score was equivalent between the three different types of stents (data not shown). Animals were sacrificed at four weeks after formalin fixation. Each group consisted of five rabbits (10 stents) as follows: 1) uncoated bare metal stents, 2) polymer only-coated stents, and 3) polymer-coated stents containing RUS-3108.
Serial cross sections (5 µm) were obtained from the proximal, mid, and distal thirds of the stented arterial segments and stained with Movat-pentachrome stain. Computerised morphometry (Scion Image software, Scion Corp., Frederick, MD, USA) was performed to determine intimal cross-sectional area (CSA), total vessel CSA (area within the external elastic lamina), and lumen area. The maximum intimal CSA in each vessel and the mean intimal CSA in the segment (n=3 sections per segment) were used for statistical analysis. Intraluminal thrombus was not included in the intimal hyperplasia measurements.
STATISTICAL ANALYSIS
All measurements are expressed as mean ± SD. Student’s t-test was used for comparison between two groups while analysis of variance (ANOVA) was performed for multiple comparisons. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P < 0.05.
Results
Induction of perlecan by RUS3108
RUS3108-treated SMC showed increased intracellular expression of perlecan as shown by immunohistochemistry (Figure 1).
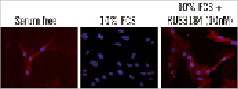
Figure 1. Immunostaining for perlecan in SMCs. Red: perlecan, blue: DAPI (nuclei), and green: α-actin.
Interestingly, RUS3108 untreated, but serum-starved cells showed an endogenous over-expression of perlecan, which was inhibited when cells were grown in serum-containing media.
In vitro effects of RUS3108 on SMC
RUS3108 inhibited SMC proliferation by 60% (p<0.05) at a dose of 1 µM RUS3108 (Figure 2, panel A).
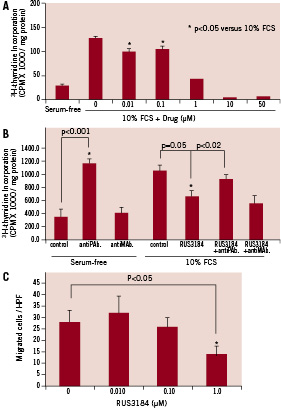
Figure 2. SMCs proliferation. A) Arterial SMCs were treated with or without RUS3108 for 48 hours and 3H-thymidine was added for 24 hours. There was ≈60% inhibition of proliferation at 1 µM of RUS3108. B) Arterial SMC were incubated in 10% FCS with RUS3108 (1 µM) in the presence or absence of 10 µg/ml of anti-perlecan antibody (antiPAb.) or mouse IgG (antiMAb.) for 48 hours and 3H-thymidine was added for 24 hours. There was an approximately 40% inhibition of thymidine incorporation with RUS3108. Perlecan antibody reversed the inhibitory effects of perlecan inducer on cell proliferation. In serum starved conditions (without RUS3108), perlecan antibody stimulated thymidine incorporation presumably due to inhibition of endogenous perlecan. A non-specific antiMAb had no effects. C) Confluent SMCs were incubated for 48 hours in 10% FCS with or without increasing doses of RUS3108. Cells were trypsinised and re-suspended in serum-free medium containing drug and added to the upper chamber of a micro-chemotaxis chamber that contained PDGF in the lower chamber. Cells that migrated to the lower side of the filter were counted. RUS-3108 significantly inhibited cells migration at 1 µM concentration. Data are mean ± SD. All experiments were performed in triplicates.
Higher doses (10 and 50 µM) were found to be toxic to SMC. When the experiment was repeated with a perlecan antibody, the antibody reversed the inhibitory effects of RUS3108 indicating the specificity of the drug and the specific effects of perlecan on cell proliferation (Figure 2, panel B). A non-specific control murine monoclonal antibody had no effects on cell proliferation. Furthermore, as shown in Figure 2C, RUS-3108 significantly inhibited cells migration at a dose of 1 µM.
In vitro effects of RUS3108 on endothelial cells
In contrast to its effects on SMC, RUS3108 was found to be mitogenic to EC. At a dose of 1 µM, EC DNA synthesis was increased by 22% (p<0.02) (Figure 3).
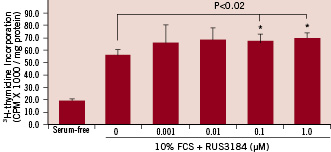
Figure 3. EC proliferation. Bovine aortic ECs (2x104 cells/well) were seeded in 24 well plates and incubated overnight. Cells were treated with increasing doses of RUS3108 for 48 hours and 3H-thymidine was added 24 hours prior to harvest. There was a modest 22% and 25% increase in cell proliferation at 0.1 µM and 1 µM drug concentrations, respectively. Data are mean ± SD. All experiments were performed in triplicates.
Effect of RUS3108 on MMP activity
As shown in Figure 4A, RUS3108 (1 µM) inhibited SMC MMP-9 activity but not MMP-2. The inhibition of MMP-9 activity could be completely reversed by the addition of a perlecan antibody.
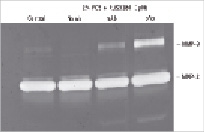
Figure 4. SMC migration. A) SMCs were grown to confluency in 96 well plates and RUS3108 (1µM) was added in 100 µl of medium containing 5% FCS in the presence or absence of a control murine IgG antibody (mAb) or anti-perlecan antibody (pAb) and incubated for 72 hours. Media was collected, mixed with 4 x sample buffer and analysed by gelatin zymography. RUS3108 inhibited MMP-9 activity which could be reversed by the anti-perlecan antibody. B) Confluent ECs were incubated in DMEM containing 2% FCS and increasing concentrations of perlecan inducer for four days. Media was collected, mixed with 4 x sample buffer and analysed by gelatin zymography. In a dose dependent manner, RUS3108 inhibited both MMP-9 and MMP-2 activity.
Effects of RUS3108 on SMC differentiation
SMembryonic is a marker of dedifferentiated state. In serum-free conditions, SMC expressed α-actin only (Figure 5, green), indicating a differentiated or quiescent phenotype. In serum-stimulated conditions, SMC demonstrated a more dedifferentiated phenotype with increased SMemb expression and decreased α-actin expression (Figure 5, mid panel). Serum-treated cells that were also treated with RUS3108 showed no SMembryonic staining similar to serum starved conditions, indicating the role of perlecan induction in the maintenance of cell quiescence.
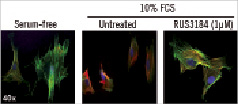
Figure 5. Immunostaining for SMembryonic-Cy3-labelled (red), α-actin-FITC-labelled (green), and nuclei (DAPI - blue). SMembryonic is a cell marker for non-differentiated cells (e.g., proliferating cells). RUS3108 treated cells did not express this dedifferentiation marker suggesting the induction of SMC quiescence.
Inhibition of in-stent intimal hyperplasia by RUS3108-eluting stents
All animals survived the procedure and the 4-week follow-up period. As shown in Figure 5, the deployment of RUS3108-eluting stents resulted in 69% reduction in in-stent intimal CSA (0.31±0.27 mm2 as compared to 1.0±0.31 mm2 and 1.25±0.51 mm2 in the bare metal and polymer-only coated stent groups, respectively (p<0.0001). Figure 6A illustrates representative cross sections from each of the groups showing the significant inhibition of in-stent intimal hyperplasia.
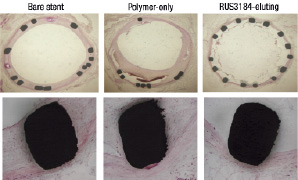
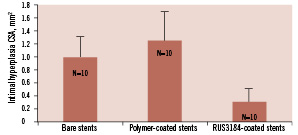
Figure 6. Neo-intimal cross sectional area (CSA) one month after iliac artery stenting in three different groups (n=10/group): 1) bare metal stents, 2) polymer-only coated stents, and 3) polymer with RUS3108-eluting stents. CSA was decreased by ≈70% by the elution of RUS3108. * p<0.0001 versus bare metal and polymer-only stents.
Additional histologic features were also evident in the two polymer stent groups. The polymer stent (no drug) showed marked inflammatory changes. There were prominent spaces around the stent struts that contained large number of necrotic cells as well as inflammatory cells in the vessel wall (Figure 7A). The RUS3108-eluting stents showed less marked changes of vessel wall inflammation with occasional necrotic SMC, scattered lymphocytes and rare giant cells. In addition, the majority of cross sections from the RUS3108-eluting stents showed evidence of intraluminal thrombus adherent to stent struts that had no or very minimal intimal hyperplasia (Figure 7B).
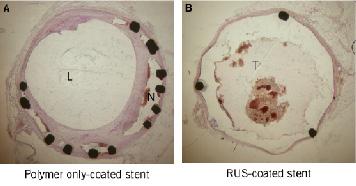
Figure 7. Representative cross sections from the three treated group showing significant inhibition of intimal hyperplasia and no vessel wall toxicity. L: lumen, N: neo-intima, T: thrombus.
Discussion
In the present study we describe the in vitro and in vivo effects of a novel perlecan-inducing compound. The induction of perlecan in SMC resulted in maintaining a differentiated phenotype and significant inhibition of cell proliferation, migration and MMP-9 activity. In contrast, the induction of perlecan in EC resulted in modest stimulation of proliferation. These in vitro characteristics combined with the known anti-thrombotic properties16 of perlecan are ideal for the prevention of in-stent restenosis. Thus, in a rabbit iliac model, perlecan-eluting stents showed a significant, 70%, reduction in intimal CSA, as compared to bare metal stents.
Our immunohistochemistry data prove that this novel perlecan-inducing compound can induce the expression of perlecan in SMC. Furthermore, the effects on SMC proliferation and MMP activity were reversed by a specific perlecan antibody indicating the specificity of RUS3108 to the induction of perlecan and that RUS3108 in vitro effects are perlecan-mediated.
Perlecan has a number of complex biologic effects that are dependent on cell type and the environment. In SMC, perlecan has been shown to be a potent inhibitor of adhesion14, migration15, proliferation12,13, and thrombus formation16. However, the mechanism(s) by which perlecan inhibits SMC activity has not been fully explored. Walker et al suggested a mechanism via up-regulation of the expression of focal adhesion kinase-related non-kinase (FRNK), a SMC-specific endogenous inhibitor of FAK, which subsequently suppresses FAK-mediated and ERK1/2-dependent SMC growth signals19. We have demonstrated that treatment with RUS 3108 lowered FAK and down regulate phosphorylation of PTEN which we propose as the pathway downstream perlecan signalling (Figure 1c).
In contrary to its inhibitory effect on SMC, perlecan appears to be a potent pro-angiogenic agent9. This duality of function depending on cell type may seem paradoxical2. There are several possible explanations. First, perlecan produced by SMC and EC may differ in heparan sulphate amount and/or composition. Differences in the heparan sulphate glycosaminoglycans (HSGAG) side chains directly affect cell signalling or through interactions with heparin-binding growth factors, particularly FGF-2 and its receptor20,21. Second, perlecan may target different genes in different cell types. For example, COP-1, a member of the CCN family of heparin-regulated genes, is specifically induced by perlecan in SMC but not in EC, and has anti-proliferative activity22. Third, the activity of the FGF family of growth factors is modulated by HSGAG, which is found both in the ECM and on the cell surface. Differences in FGF-mediated proliferation in SMC and EC appear to be related to the distinct cell surface HSGAG of the two cell types23.
Differences in the ECM of SMC and EC also profoundly affect cellular behaviour. The sequestering of heparin-binding growth factors such as FGF by perlecan in the SMC ECM may be an important factor in inhibiting SMC proliferation. Perlecan may behave differently in the presence of other matrix compounds such as fibronectin24. Thus, the bioactivity of HSPG such as perlecan is dependent on its cell origin, subtle changes in structure (including the size of the heparin side chains and its glycosolation status), the concentration and binding kinetics of the growth factor, and the expression of a specific receptor and its isoforms21,25.
Our in vitro studies provided an additional interesting observation. Serum-starved SMC synthesise perlecan, indicating the role of perlecan in maintaining quiescence under stress conditions. Under serum starvation, cell survival is probably dependent upon the autocrine effects of endogenous growth factors. Possibly, perlecan over-expression causes the sequestration of these growth factors. Therefore, blocking perlecan may promote the effects of growth factors. This observation emphasises the crucial role of perlecan in maintaining SMC quiescence under stress conditions such as serum starvation.
Our in vivo data show a significant inhibition (70%) of neo-intima CSA when RUS3108-eluting stents were deployed as compared to bare metal stents. Previous in vivo studies showed down-regulation of perlecan expression in the vessel wall immediately after and seven days after arterial vascular injury when SMC proliferation was maximal26. Similar studies in rabbits using the rapamycin- and paclitaxel-eluting stents showed only 45-50% reduction27,28 and 36-49% reduction29, respectively. Furthermore, histological findings suggested incomplete healing in paclitaxel-containing stents consisting of persistent intimal fibrin deposition, intra-intimal haemorrhage, and increased intimal and adventitial inflammation29. RUS3108-treated arteries showed modest evidence of cell necrosis and inflammation in vessel wall. This may be attributed to the dual and opposing effects of perlecan on SMC and EC promoting rapid healing.
In addition, the polymer-only stent group showed quite marked inflammatory changes in the vessel wall in addition to spaces surrounding the stent struts containing prominent collections of necrotic cells. These effects appear to be due predominately to the thrombogenic and inflammatory properties of the polymer that was used to coat the stent struts. Despite the potent inhibitory effects on intimal hyperplasia in the RUS3108-treated arteries, there was thrombus adherent to the stent struts in the majority of the sections. The thrombus formation was apparent almost entirely in the RUS3108-eluting stents. The thrombus was adherent to stent struts that had essentially no intimal hyperplasia. It is well known that stent struts are thrombogenic in the early period after placement of bare metal stents prior to the formation of the intimal hyperplasia covering the stent and particularly, in drug-eluting stents that suppress intimal hyperplasia. In addition, certain polymers used in drug-eluting stents have been shown to contribute to stent thrombosis30. Of interest, perlecan has actually been shown to be anti-thrombotic16. Thus, it is possible that using the perlecan-inducing compound with a more biocompatible polymer on the stent would significantly lower the thrombus propensity evident in this study.
The purpose of this study was to assess the effects of RUS3108 on intimal hyperplasia, one of the limitations of the preliminary study is that we might have used higher drug concentrations as compared to concentration that were used in other drug-eluting stents, and further experiments should be performed for comparison of intimal hyperplasia response and effect on endothelial coverage between different drugs as well as on lower concentrations of RUS3108.
In conclusion, a novel compound that induces the expression of perlecan resulted in significant inhibition of SMC activity and maintenance of differentiated SMC phenotype while modestly promoting EC proliferation. The deployment of RUS3108-eluting stents in rabbit iliac arteries resulted in significant inhibition of in-stent intimal hyperplasia. The development of more biocompatible, less inflammatory polymers for eluting RUS3108 may serve as a promising modality for the prevention of in-stent restenosis.

