Abstract
Aims: We conducted a prospective observational study using a course of steroids and antihistamines to treat a cohort of patients who developed skin reactions to clopidogrel, to assess whether dual antiplatelet therapy could be continued in an outpatient setting.
Methods and results: This study included 2,701 patients who underwent percutaneous coronary intervention (PCI) at our centre over a 23 month period. Patients with skin reactions to clopidogrel were identified and then commenced on five days oral prednisolone (30mg/od) and chlorpheniramine (4 mg/tds) for seven days. A subsequent telephone survey was performed to evaluate a number of variables. The probability of the adverse reaction being secondary to clopidogrel was assessed using the Naranjo adverse drug reaction probability scale. Twenty (0.7%) patients were identified who developed adverse skin reactions to clopidogrel. There was complete resolution seen in the majority (89%) of patients within an average of 3.2 days following treatment. One patient had partial resolution, and one had no response to treatment, but both were able to continue clopidogrel.
Conclusions: We propose a novel, safe and effective way of managing clopidogrel-induced skin reactions using a short course of prednisolone and chlorpheniramine, without stopping or substituting clopidogrel.
Introduction
Over the past 30 years percutaneous coronary intervention (PCI) and stent implantation have become the dominant mode of treatment for patients with obstructive coronary artery disease (CAD). Advances in operator expertise, equipment and adjunctive pharmacological treatment have made it possible to treat complex CAD, including left main stem, bifurcation and multivessel disease. The widespread use of drug-eluting stents (DES) has dramatically reduced the incidence of restenosis and the need for repeat revascularisation compared to balloon angioplasty alone.1,2 Improvements in adjunctive pharmacology have also improved outcomes, particularly through a reduction in the risk of acute and subacute stent thrombosis. Clopidogrel and aspirin are currently the optimal antiplatelet regimen during and after coronary artery stenting. In some situations it may prove difficult to use this combination, particularly in those patients who develop a skin rash or allergy to clopidogrel.
The use of aspirin alone, aspirin and ticlopidine (another thienopyridine derivative), or full anticoagulation have all demonstrated a reduction in the incidence of stent thrombosis.3-5 Dual antiplatelet therapy is associated with significantly less stent thrombosis than aspirin alone and with less stent thrombosis and fewer bleeding events than the combination of aspirin and anticoagulation.3-5 Clopidogrel has now superseded ticlopidine as the thienopyridine derivative of choice, because it has a faster onset of action, superior efficacy in preventing stent thrombosis and an improved side-effect profile.6-8 Ticlopidine is associated with the potentially life-threatening complications of neutropenia and thrombotic thrombocytopenic purpura in 2-2.5% of cases. Clopidogrel has a synergistic effect with aspirin, both in terms of platelet inhibition and in improving clinical outcomes. Clopidogrel has been shown to provide incremental benefit to aspirin alone in acute coronary syndromes (ACS),9 ST-segment elevation myocardial infarction (STEMI)10,11 and following elective and emergency percutaneous coronary intervention (PCI).12,13 Our hospital policy is for 12 months dual antiplatelet therapy with aspirin and clopidogrel following an ACS or after insertion of a DES, and at least one month following insertion of a bare metal stent (BMS) in line with the European Society of Cardiology guidelines.14
Despite the routine use of dual antiplatelet therapy there are ongoing concerns as to the risk of acute and subacute stent thrombosis. Premature discontinuation of antiplatelet therapy is the most powerful known risk factor for stent thrombosis with a hazard ratio of 89.8.15 Alternative treatment regimens, including the use of warfarin,3 low molecular weight heparin16-18 or anti-X agents,19,20 are less effective at preventing stent thrombosis. Factors which influence the likelihood of clopidogrel discontinuation, including skin reactions or allergies, are consequently of paramount importance. Recently described desensitisation procedures are time-consuming, may require prior admission to hospital and are unsuitable for patients already established on maintenance therapy.21-24 There are several novel thienopyridines in development that could provide equivalent protection when available.25
Potential skin reactions to clopidogrel have been reported in up to 6% of patients, requiring drug discontinuation in 0.7 to 0.9%.6,8,23 Other reported adverse reactions include gastro-intestinal upset, liver function abnormalities, thrombotic thrombocytopenic purpura, aplastic anaemia, neutropenia (in 0.1% of patients) and serum sickness.26,27 In the CAPRIE study potential clopidogrel-induced rashes occurred throughout the study period (mean follow-up 1.91 years) and the incidence was independent of age or gender.6
We conducted a prospective observational study to:
a) Assess the rates of skin rash (drug eruptions) to clopidogrel therapy in a contemporary cohort of patients undergoing percutaneous coronary intervention and stent implantation, and
b) Evaluate a potential treatment strategy using steroids and antihistamines to allow continuation of clopidogrel in an outpatient setting.
Methodology
This prospective study included patients who underwent single or multivessel PCI at our institution over a 23 month period, between January 2006 and November 2007. Clopidogrel cards were given to all of the patients following PCI to remind them of the importance of continuing dual antiplatelet therapy and with a contact phone number should they develop any adverse reactions.
Outpatients with skin reactions to clopidogrel were identified following presentation to either their local general practitioner (GP) or through direct contact with our department, as advised at the time of their original procedure. These patients were then discussed with their interventionalist and commenced on five days oral prednisolone (30 mg/od) and chlorpheniramine (4mg/tds) for seven days, with subsequent follow-up at their GP practice.
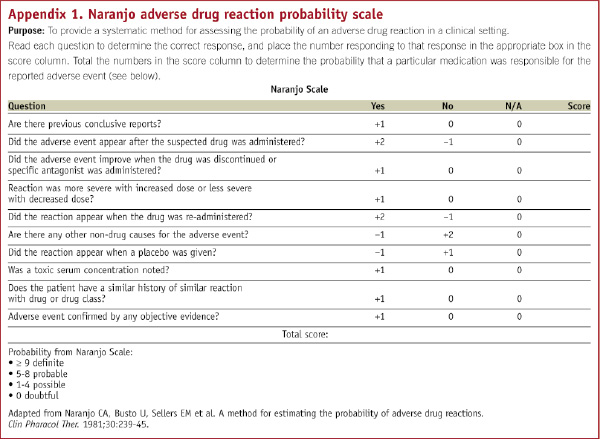
Inpatients with an adverse skin reaction to clopidogrel treatment were reviewed by their interventionalist and then commenced on the same combination. Treatment with dual antiplatelet therapy was continued throughout. A subsequent telephone survey was performed to evaluate: procedures performed, onset of adverse reaction, details of the drug eruptions, treatment given or changed, improvement seen, any recurrence, hospital admissions and history of discontinuation of clopidogrel. The probability of the adverse reaction being secondary to clopidogrel was assessed using the Naranjo adverse drug reaction probability scale.28 (Appendix 1)
Results
During the study period 2,701 patients underwent PCI. Twenty (0.7%) patients were identified who developed adverse skin reactions to clopidogrel. Nineteen patients were available for follow-up (nine males and 10 females); one patient could not be contacted. The average patient age was 67.2 years (29-85 years). Seventeen (89%) of the patients had DES and two (11%) had (BMS). Four (21%) patients had staged procedures. The reaction developed between one to 21 days (average 4.5 days, median of three days) after commencing clopidogrel. It was generalised in 17 (89%) and localised (hands and chest) in two (11%) of the patients. Only two (11%) patients had blood tests performed, which did not reveal any haematological abnormalities.
All patients were treated with five days oral prednisolone and chlorpheniramine for a period of seven days, except two cases that responded to topical steroid cream and chlorpheniramine only. None of the patients required hospital admission. There was complete resolution seen in the majority (89%) of patients within one to 14 days (average 3.2 days) following treatment. One patient had partial resolution, but was able to continue clopidogrel. One patient had no response to treatment of their localised skin reaction, but did not discontinue clopidogrel. The rash resolved as soon as clopidogrel was stopped after the pre-specified time period of 12 months. The mean Naranjo adverse drug reaction probability scale was 6 (range 3-7), consistent with a probable association of clopidogrel in 17 (89%) and possible association in two patients (11%).28 No recurrences of the rash or side-effects to the treatment were reported.
Discussion
Antiplatelet agents have a pivotal role to play in the prevention and management of atherothrombosis. Stent thrombosis is an important, life-threatening complication of coronary stent placement that has been associated with mortality rates of up to 45%.15 Clopidogrel bisulphate, an oral thienopyridine derivative, prevents platelet aggregation by non-competitive inhibition of adenosine diphosphate receptors (ADP) and is considered essential to reduce the risk of stent thrombosis in combination with aspirin. It is reported to have a safety profile comparable or superior to aspirin and is better tolerated than ticlopidine.
However, several adverse reactions have been documented with clopidogrel, the commonest being cutaneous rashes, pruritis and gastrointestinal upset. Rarer adverse reactions including thrombotic thrombocytopenic purpura, haemolytic uraemic syndrome, aplastic anaemia, and severe hypersensitivity can be life threatening.26,29 These may necessitate the discontinuation of clopidogrel with a resulting increased risk of stent thrombosis. The alternative thienopyridine, ticlopidine, is more expensive, requires twice-daily dosing and is associated with a more frequent and serious side-effect profile, limiting its clinical usefulness.
In our series we found that, out of 2,701 PCI procedures performed over a 23 month period, only 0.7% (20) of patients reported an adverse skin reaction to clopidogrel. This rate is low compared to the 2 to 6% of skin reactions reported elsewhere.6,7,9,23 Possible reasons for this include non-reporting of milder reactions by some patients or their GPs, or by their presentation and management in another hospital. In the CAPRIE study, which included 9,577 patients on clopidogrel, only 0.26% (25 patients) of the study population had reactions considered by the investigators to be severe and 0.9% (86 patients) permanently discontinued the drug due to the rash.
The majority of patients with skin reactions presented as diffuse maculopapular eruptions, with a localised rash being less common. In our study 16 (84%) of the patients had a rash affecting the whole body, one the chest, one the abdomen and one affecting the hands. None reported mucosal reactions or fever. The mean time to onset of the rash was 4.5 days, similar to other reports.23 There was no evidence of haematological abnormalities in those patients tested. However, no immunoglobulin assays were carried out to identify immunoglobulin-E mediated allergic response. Treatment with a five day course of prednisolone and chlorpheniramine for a period of seven days allowed continuation of clopidogrel in all cases. The use of steroids and antihistamines in patients with clopidogrel-induced reactions has been reported, but these were associated with the discontinuation of clopidogrel, because of the severity of the allergic response.26,27 In our study there were no hospital admissions as a consequence of the reaction. There was complete resolution of the rash within 14 days of initiating treatment in 84% of our cohort. There was no recurrence of rash in any patient, despite some of them having a staged PCI at a later date. No side-effects to the treatment were reported.
This study shows that treatment of clopidogrel-induced drug eruptions with steroids and chlorpheniramine is safe, allows continuation of clopidogrel and leads to resolution of the rash in the majority of cases. This strategy avoids the need for drug discontinuation with the associated risks of stent thrombosis, or the need for alternative antiplatelet or antithrombotic therapies.
Limitations
However, there are a number of limitations in our single-centre study including:
1. The patients in this small non-randomised study presented with their rash to their GP or our centre. It is likely that some patients with adverse skin reactions did not inform anyone, or that their GP did not contact us, or that they presented elsewhere, so that we underestimated the prevalence of adverse drug eruptions.
2. Skin reactions can occur in response to intravenous contrast, drug eluting stents or other drugs and these are potential confounding factors. However, treatment with steroids and antihistamines is preferable to discontinuation of clopidogrel, or changing to ticlopidine. The majority of patients scored in the range consistent with a probable clopidogrel drug reaction on the Naranjo adverse drug reaction probability scale.28
3. The study is based on a telephone questionnaire survey, so there is scope for bias as the questions are pre-selected and based on patient memory.
4. In our study none of the patients had a life-threatening reaction or anaphylaxis, mandating hospitalisation. In this situation, we would have had to withdraw clopidogrel and then substitute it with another agent, such as ticlopidine.
5. There is no universally accepted scale for describing or measuring the severity of an adverse drug reaction. Assessment is largely subjective and reactions are often classified as mild, moderate or severe based on the clinical presentation. Patients with severe reactions including systemic symptoms, mucosal involvement and lab abnormalities may require hospitalisation for treatment.
6. Cytochrome P4502C19 plays a crucial role in the formation of the active metabolite of clopidogrel. Prednisolone is an inducer of cytochrome P4502C19 and the antiplatelet effect of clopidogrel may be affected during the treatment of these reactions with steroids. There is currently no accepted method of assessing clopidogrel responsiveness and this was not measured in our study, but may have important implications.
Conclusions
Clopidogrel-induced skin reactions are not rare and withdrawing clopidogrel early is high risk, especially in the era of drug eluting stents and stent thrombosis. We propose a novel, safe and effective way of managing this problem using a short course of prednisolone and chlorpheniramine, without stopping or substituting clopidogrel. We anticipate that this will provide clinicians with a practical method of addressing this problem in their daily practice.

