Introduction
The harmful effects of radiation were soon recognised after the discovery of x-rays by Wilhelm Roentgen in 18951. Increased incidences of skin cancer and leukemia confirmed the carcinogenic potential of x-rays in the early twentieth century2. Interventional cardiologists experience frequent radiation exposure through fluoroscopy. Interventional cardiology procedures performed via the radial approach are associated with longer fluoroscopy times and greater cumulative scatter radiation to the operator and staff3. This approach is gaining popularity and exceeds 90% of cases in dedicated centres4. The cumulative risk associated with a lifetime of exposure could become significant5,6. The lower torso (from the umbilicus and down) acts as a source of scatter radiation to the operator and is not routinely shielded. We developed a uniquely designed, non-disposable lead attenuating material that could shield this region and significantly reduce the radiation scatter exposure to an operator in the catheterisation laboratory.
Device description
A rectangular lead attenuator was developed to shield the lower torso from radiation scatter exposure during radial procedures.
Technical specifications
A folded lead apron (UltraRay Medical Products Inc., Oakville, ON, Canada) was used to create a rectangular shielded area with dimensions of 60×100 cm, and an attenuation equivalent to 0.50 mm of lead (Figure 1).
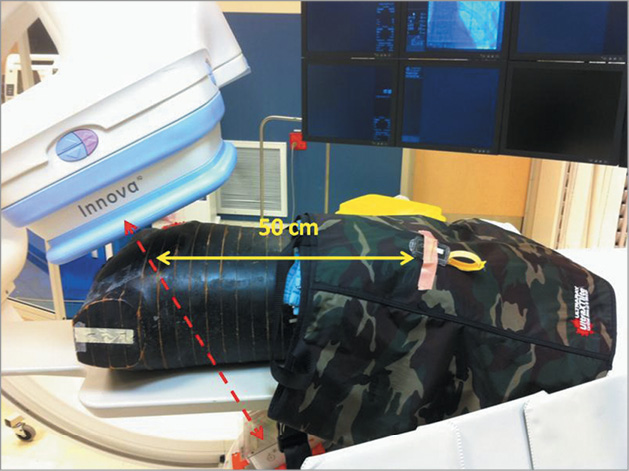
Figure 1. RANDO® phantom on the catheterisation laboratory table. The phantom is covered by the lead apron starting at the umbilicus and extending over the legs. Personnel dosimeters (Landauer, Inc., Glenwood, IL, USA) were deployed underneath and above the lead 50 cm from the centre of the radiation beam as well as 100 cm from the beam (not shown).
Indications for use
This lead apron is intended for use during radial angiography interventions.
Preclinical experience
Studies were performed using the RANDO® phantom (The Phantom Laboratory, Salem, NY, USA), which mimics the x-ray attenuation and scatter produced by the human body. It is composed of a natural human skeleton embedded with a proprietary urethane formulation that simulates muscle tissue and closely mimics the density of lungs. Fluoroscopy was performed using an Innova 2121-IQ biplane catheterisation system (General Electric Healthcare, Buc, France). InLight® personnel dosimeters (Landauer, Inc., Glenwood, IL, USA) were used to measure all doses. These dosimeters are sensitive to scattered radiation coming from all angles7.
For each experiment, scattered radiation dose measurements were performed first without the rectangular lead shield, and then with the shield covering the phantom from a point corresponding to the umbilicus and extending for 60 cm towards the feet. There was no interference with visualisation. Scattered radiation was measured for all experiments at the same two locations. Location I was on top of the lead rectangle covering the phantom 50 cm from the primary radiation beam. Location II corresponded to the location of the operator and was 100 cm from the radiation beam 30 cm above the table. Fluoroscopy exposure was performed until the dose area product (DAP) of the primary beam reached a value of 20,000 cGy cm2, as indicated by the Innova, requiring an exposure time of approximately 30 minutes. Irradiation was repeated each time in three projections: straight anteroposterior (AP) with 25 degrees cranial, left anterior oblique (LAO) 39 degrees with cranial 26 degree and caudal 44 degree positions. The total examination dose released by the fluoroscopic source was calculated as DAP, in cGy cm2 units. Data are presented as mean±standard deviation. Comparison between groups was performed using the t-test for continuous variables.
A reduction in scattered radiation was recorded in all studies with the use of the lead drape.
Dosimeter readings were combined for all three aforementioned projections yielding values at both dose-monitoring locations with and without the attenuator. The exposures without the attenuator yielded values of 3.03±0.27 and 1.56±0.2 mSv at Locations I (50 cm) and II (100 cm), respectively. The exposures with the attenuator yielded values of 0.15±0.22 and 0.21±0.11 mSv at Locations I and II, respectively. The dosimeter between the attenuator and the phantom exactly underneath Location I yielded a value 2.86±0.4 mSv (Table 1).
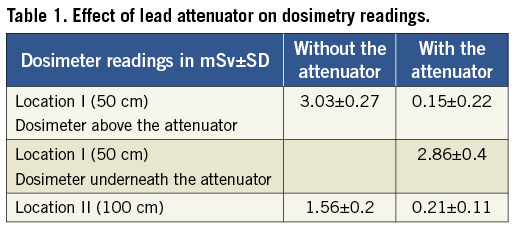
These studies demonstrate that a rectangular attenuator significantly reduced the operator’s exposure to scattered radiation (93% at 50 cm; 86% at 100 cm). Additionally, we observed no increase in radiation patterns underneath the lead attenuator. This novel and simple solution could be used in all radial artery approach procedures. Operators using the radial artery compared to a femoral artery approach would benefit particularly from the attenuator since they experience a higher radiation scatter due to standing in close proximity to a higher scattered radiation zone. Using current radiation safety techniques, some operators receive an annual dose close to 50 mSv per year. This exposure is associated with an incremental increase in mortality risk (0.2% per year), and above the lifetime risk of a spontaneously occurring fatal cancer5. Procedures performed through the radial artery are on average longer and cause higher radiation exposure to patients and operators4. Widespread adoption of this technique may lead to even higher radiation exposure and higher risks of cancer.
Recently, a disposable bismuth-barium radiation shield drape (RadPad®, 35 USD; Worldwide Innovations and Technologies, Overland Park, KS, USA) was shown to reduce the dose of operator radiation by 23%8. A 35×40 cm drape shield was positioned on the patient arm around the area of sheath insertion and extended medially to the patient body. Our study showed much larger reductions (>3x) in operator radiation. This may be due to several reasons, including the location of shielding and the shielded area (100×60 cm), which provides a >4-fold larger area of protection.
We have now developed a rectangular lead attenuator (UltraRay Medical Products Inc., Oakville, ON, Canada) for clinical studies, which has the same dimensions as the folded apron used in these phantom studies. Since the attenuator is inserted in a sterile plastic bag, it is reusable (Figure 2). If the femoral approach is needed, the attenuator can be easily repositioned while maintaining a sterile groin. Lange et al9 showed that a pelvic lead shielding (MAVIG GmbH, Munich, Germany, 1,500 USD) can reduce the scattered radiation mainly in femoral more than in radial procedures. Our attenuator is more effective due to its larger area (there are no holes for femoral puncture or upper diagonally cut abdominal portion), but can be easily repositioned if fluoroscopy is needed for the lower abdomen or groin. Since the lead rectangle is in a sterile nylon bag and both groins are routinely prepared for catheterisation, the attenuator could be promptly removed in the event of emergency requirement of femoral puncture (Figure 2).

Figure 2. Clinical example of lead rectangle in use for a left radial procedure. The lead attenuator was inserted into a sterile nylon bag. After cleaning both groins, sterile towels were used to cover the groin area, and positioned on top of the lead attenuator and then covered by a sterile drape. If an urgent femoral puncture is needed, the attenuator can be removed promptly while maintaining sterility.
Limitations
This study was performed using a phantom and only tested three of the multiple possible views, although the results were consistent in all of the views tested. We used a single rectangle that best accommodates radial procedures; two smaller rectangles, 30×100 cm each, could theoretically provide similar protection if radial and femoral procedures are required simultaneously. A femoral puncture can be done between the rectangles and these can be repositioned for aortoiliofemoral angiography. The impact of the attenuator on patient and personnel radiation patterns should be tested further in clinical studies.
Conclusion
This novel rectangular scatter attenuator significantly reduces radiation scatter when used with an anthropomorphic phantom that mimics radial artery catheterisation procedures in patients. A clinical study is currently ongoing to provide clinical validation of this approach.
Conflict of interest statement
N. Robert and A.B. Osherov have applied for a patent based on the aforementioned device. The other authors have no conflicts of interest to declare.

