Abstract
Percutaneous coronary intervention (PCI) has been most commonly guided by coronary angiography. However, to overcome the inherent limitations of conventional coronary angiography, there has been an increasing interest in the adjunctive tools of intracoronary imaging for PCI guidance. Recently, optical coherence tomography (OCT) has garnered substantial attention as a valid intravascular imaging modality for guiding PCI. However, despite the unparalleled high-resolution imaging capability of OCT, which offers detailed anatomical information on coronary lesion morphology and PCI optimisation, its broad application in routine PCI practice remains limited. Several factors may have curtailed the widespread adoption of OCT-guided PCI in daily practice, including the transitional challenge from intravascular ultrasound (IVUS), the experienced skill required for image acquisition and interpretation, the lack of a uniform algorithm for OCT-guided PCI optimisation, and the limited clinical evidence. Herein, we provide an in-depth review of OCT-guided PCI, involving the technical aspects, optimal strategies for OCT-guided PCI, and the wide application of OCT-guided PCI in various anatomical subsets. Special attention is given to the latest clinical evidence from recent randomised clinical trials with respect to OCT-guided PCI.
Several limitations of conventional coronary angiography as the primary diagnostic tool to assess obstructive coronary artery disease (CAD) have been recognised1. Coronary angiography provides only a two-dimensional lumenogram of the coronary tree, thus making it difficult to comprehensively assess plaque or vessel characteristics of atherosclerotic CAD. To overcome such inherent limitations, intravascular imaging modalities such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) have emerged as valuable diagnostic tools for better understanding the anatomical characteristics of CAD and guiding percutaneous coronary intervention (PCI)2.
IVUS and OCT can provide more detailed insights into the morphology of the vessel wall, lumen, and atherosclerotic plaque; optimise stent implantation (e.g., appropriate sizing, optimal stent landing zone, adequate apposition and expansion); and minimise stent-related procedural complications (e.g., exclusion of edge dissection and haematoma)345, thereby reducing the risk of adverse cardiovascular events including stent thrombosis, myocardial infarction (MI), death from cardiac causes, and repeat revascularisation. This clinical benefit of intravascular imaging compared with coronary angiography alone has been supported by several clinical studies, including observational registries, randomised controlled trials (RCTs), and meta-analyses678910111213141516171819.
Despite the strong evidence in favour of intravascular imaging-guided PCI over angiography-guided PCI, the widespread adoption of these techniques in daily PCI practice is still limited. Several factors may contribute to this underutilisation, including a lack of familiarity and experience with the imaging equipment and image interpretation, the lack of standardised imaging-guided PCI algorithms, concerns regarding a potential increase of procedural time and the impact on workflow efficiency in the catheterisation laboratory, and the different reimbursement policies52021.
Recently, OCT has risen to prominence as an essential adjunct for PCI guidance22, but its widespread adoption into routine PCI practice is still limited. In this review, we present a comprehensive overview of OCT-guided PCI involving several important technical and procedural aspects, and we also provide an in-depth review of clinical evidence from recent clinical studies.
OCT imaging: technical considerations
TECHNICAL DIFFERENCES BETWEEN OCT AND IVUS
Key technical differences, along with the relative strengths and weaknesses of IVUS and OCT, are summarised in Supplementary Table 1. OCT can provide a higher resolution (nearly 10 times greater) and faster image acquisition compared to IVUS, but it requires blood clearance for image acquisition. On the other hand, OCT has lower tissue penetration (except in calcified plaque) and potential imaging attenuation by red thrombus, lipid, and necrotic cores. Technically, OCT provides a clearer interface between the lumen and intima surface, and thus, OCT has been shown to be more precise for delineating lumen contours, while IVUS more accurately identifies vessel contours, offering full-thickness visibility of the vessel wall in non-calcified vessels45. Due to these differences, the choice between IVUS and OCT should be based on in-laboratory availability and operator experience and discretion, as well as specific clinical or lesion characteristics. The selection criteria for imaging modality according to clinical or anatomical characteristics are summarised in Table 1.
Table 1. Imaging modality choice according to specific clinical or lesion subsets or plaque characteristics.
| IVUS | Specific subset | OCT |
|---|---|---|
| Clinical subset | ||
| + | ACS culprit lesions | ++ |
| − | INOCA or MINOCA | ++ |
| ++ | SCAD | − |
| ++ | Renal insufficiency | − |
| Lesion subset | ||
| ++ | Left main disease | +* |
| ++ | Bifurcation lesions | ++ |
| ++ | Ostial lesions | − |
| ++ | CTO lesions | + |
| + | ISR lesions | ++ |
| + | Calcified lesions | ++ |
| Plaque or stent characteristics | ||
| + | Ruptured plaque or thrombus | ++ |
| + | Neoatherosclerosis | ++ |
| + | Stent apposition | ++ |
| + | Stent edge dissection | ++ |
| − | TCFA | ++ |
| *OCT assessment of left main lesions is limited to mid-distal left main. +: good assessment or indication; ++: excellent assessment or indication; -: poor assessment or indication. ACS: acute coronary syndrome; CTO: chronic total occlusion; INOCA: ischaemia with no obstructive coronary arteries; ISR: in-stent restenosis; IVUS: intravascular ultrasound; MINOCA: myocardial infarction with no obstructive coronary arteries; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; SCAD: spontaneous coronary artery dissection; TCFA: thin-cap fibroatheroma | ||
IMAGING ACQUISITION AND INTERPRETATION
Currently, the most widely used OCT systems are the OPTIS system (Abbott) and the LUNAWAVE system (Terumo). Contemporary OCT technology necessitates “bloodless” imaging, as light waves are significantly attenuated by blood. In cases where delivery of the imaging catheter is anticipated to be difficult owing to tight stenoses, severe tortuosity or calcification, predilation or the use of a guide extension is frequently required. To achieve adequate blood clearance for clear imaging, proper engagement of the guiding catheter is essential, but its deep engagement should be avoided to prevent pushing back the guide during contrast delivery or causing unintended coronary dissection. After the injection of intracoronary nitroglycerine, the OCT catheter’s lens marker is positioned 10 mm distal to the target lesions. Then, a contrast dye is injected through the guiding catheter to clear blood and facilitate quality imaging while the catheter position is optimised in order to obtain sufficient blood clearance during pullback.
Standard OCT systems offer two pullback lengths (75 mm or 54 mm): (1) the 75 mm length is faster, requires less contrast, and captures 5 frames/second, and is generally used to assess plaque morphology and decide on stent size and length; (2) the 54 mm length is slower and requires more contrast but provides a higher resolution of 10 frames/second, so is better suited to stent evaluation post-PCI and can be advantageous in guidewire recrossing during bifurcation stenting23. Over the years, a variety of established companies have introduced OCT imaging systems into routine PCI practice; while the OPTIS system is most commonly used, offering comprehensive angiographic and OCT visualisation (coregistration) capabilities, the LUNAWAVE system is also used in many centres, and several new OCT systems are currently entering PCI practice. Thus, continuing education and technical expertise are essential to ensure that OCT-guided PCI can be effectively implemented across different OCT devices in contemporary PCI practice.
The interpretation of OCT images requires an algorithmic approach to identify common morphologies of atherosclerotic coronary plaques. Different plaque morphologies exhibit unique light-attenuating properties. High attenuation occurs when near-infrared light is completely absorbed, obscuring the underlying vessel structure. In contrast, low attenuation allows light to be refracted, enabling the visualisation of vessel characteristics extending towards the adventitia. Through analysing different attenuation patterns, various plaque components (e.g., fibrous plaque, red or white thrombus, lipid plaque, or calcified plaque) can be identified during OCT image interpretation2.
OCT-guided PCI: clinical evidence
Several large observational studies have demonstrated that intravascular imaging guidance, when compared with angiographic guidance, reduces the long-term risk of mortality or major ischaemic events in patients undergoing complex PCI2425. Such a benefit of intracoronary imaging for PCI guidance has been subsequently confirmed by several RCTs615262728. In light of this evidence, clinical guidelines and expert consensus suggest that both IVUS and OCT are similarly effective in guiding and optimising most PCI procedures32029. However, until recently, the data supporting the clinical use of OCT were limited in comparison with IVUS.
Previously, several observational studies showed that OCT-guided PCI was associated with better clinical outcomes compared to angiography-guided PCI91011 (Supplementary Table 2). Recently, the clinical benefits of OCT-guided PCI have been investigated in several landmark RCTs, particularly targeting complex coronary artery lesions153031, and meta-analyses of these trials have been subsequently published16171819. The trial design and key findings of the most recent RCTs and meta-analyses are summarised in Table 2 and Supplementary Table 3.
The Randomized Controlled Trial of Intravascular Imaging Guidance versus Angiography-Guidance on Clinical Outcomes after Complex Percutaneous Coronary Intervention (RENOVATE-COMPLEX-PCI) showed that, in patients with complex coronary artery lesions, imaging-guided PCI (74% of patients with IVUS and 26% with OCT) led to a lower risk of a primary composite endpoint of death from cardiac causes, target vessel-related MI, and clinically driven target vessel revascularisation (TVR) when compared to angiography-guided PCI15. In this trial, the results of the primary endpoint analysis were similar in the subgroups of patients who underwent OCT or IVUS (53% reduction of primary events with OCT and 44% reduction with IVUS compared with angiography alone).
The European Trial on Optical Coherence Tomography Optimized Bifurcation Event Reduction (OCTOBER) randomly assigned a total of 1,201 patients with complex coronary artery bifurcation lesions to OCT-guided PCI or angiography-guided PCI30. At a median follow-up of 2 years, the incidence of the primary composite endpoint of target lesion failure (TLF), defined as death from a cardiac cause, target lesion MI, or ischaemia-driven target lesion revascularisation (TLR), was significantly lower in the OCT-guided group than in the angiography-guided group (10.1% and 14.1%, respectively, hazard ratio [HR] 0.70, 95% confidence interval [CI]: 0.50-0.98; p=0.035).
The ILUMIEN IV: OPTIMAL PCI trial randomly assigned a total of 2,487 patients with medication-treated diabetes or complex coronary artery lesions to OCT-guided PCI or angiography-guided PCI31. A final blinded OCT procedure was performed in patients in the angiography group. As one of the primary efficacy endpoints, OCT guidance resulted in a larger minimum stent area (MSA) after PCI than angiography guidance (5.72±2.04 mm2 in the OCT group and 5.36±1.87 mm2 in the angiography group). However, this mechanistic gain in MSA did not translate into a significant reduction of the primary clinical endpoint of target vessel failure (TVF) at 2 years (7.4% and 8.2%, respectively, HR 0.90, 95% CI: 0.67-1.19; p=0.45). The incidence of stent thrombosis (definite or probable) within 2 years was lower in the OCT group than in the angiography group (0.5% and 1.4%, respectively; p=0.02).
Although clinical guidelines and expert consensus suggest equivalent effectiveness of both IVUS and OCT for PCI guidance32029, direct comparative trials of the two imaging modalities were limited. In the ILUMIEN III trial, MSA and stent expansion with OCT-guided PCI were comparable to those with IVUS-guided PCI; however, neither were significantly larger than with angiography-guided PCI, without differences in clinical outcomes32. In the OPtical Frequency Domain Imaging vs. INtravascular Ultrasound in Percutaneous Coronary InterventiON (OPINION) study, OCT-guided PCI was non-inferior to IVUS-guided PCI with respect to TVF at 1 year13. However, these studies were underpowered for relevant clinical outcomes and included low-risk patients with simple coronary lesions.
The Optical Coherence Tomography Versus Intravascular Ultrasound Guided Percutaneous Coronary Intervention (OCTIVUS) study was a pragmatic randomised trial that conducted a direct comparison between OCT-guided and IVUS-guided PCI in patients with a broad range of coronary artery lesions33. The primary results of OCTIVUS demonstrated that OCT-guided PCI was non-inferior to IVUS-guided PCI with respect to a primary composite endpoint of TVF at 1 year (2.5% and 3.1%, respectively; p<0.001 for non-inferiority). Although the amount of contrast dye used was higher in the OCT-guided group than in the IVUS-guided group, the incidence of contrast-induced nephropathy was similar in both groups (1.4% in the OCT group vs 1.5% in the IVUS group; p=0.85). The incidence of major procedural adverse events was lower in the OCT group than in the IVUS group (2.2% vs 3.7%; p=0.047). However, there were no events directly caused by imaging procedures in either group.
Subsequently, several meta-analyses have consistently shown that imaging-guided PCI with OCT or IVUS was associated with reduced risks of major cardiovascular events or mortality compared with angiography-guided PCI16171819. There were no significant differences in effectiveness nor safety outcomes between IVUS-guided PCI and OCT-guided PCI.
Contemporary and updated clinical guidelines regarding imaging-guided PCI are summarised in Table 3. Both European and US guidelines recommend the consideration of intravascular imaging, either IVUS or OCT, particularly for optimising stent implantation in selected patients and in complex coronary lesions2029. OCT is considered a reasonable alternative to IVUS for procedural guidance, except in cases of ostial left main disease. Furthermore, both imaging tools are recommended for determining the mechanism of stent failure. Recently, European guidelines have also suggested that imaging-guided PCI should be considered in acute coronary syndrome (ACS) settings, particularly the use of OCT in ACS patients with ambiguous culprit lesions34.
Table 2. Randomised controlled trials and meta-analyses of OCT-guided PCI*.
| Trial (year) | Population | Trial endpoints |
|---|---|---|
| Randomised controlled trials | ||
| Habara et al (2012)12 | OCT-guided PCI (n=35) vs IVUS-guided PCI (n=35) | Stent expansion analysed by IVUS |
| DOCTORS (2016)7 | 240 NSTEMI patients: 120 OCT-guided PCI vs 120 angiography-guided PCI | Post-PCI FFR |
| OPINION (2017)13 | 414 in OCT-guided PCI group vs 415 in IVUS-guided PCI group | TVF at 1 year |
| ILUMIEN III (2016)32 | 158 OCT-guided vs 146 IVUS-guided vs 146 angiography-guided PCI | Post-PCI MSA assessed by OCT |
| ROCK II (2022)8 | 377 intravascular imaging-guided PCI (162 OCT, 215 IVUS) vs 353 angiography-guided PCI | TLF at 1 year |
| RENOVATE-COMPLEX PCI (2023)15 | 1,639 patients with complex coronary artery lesions, 2:1 randomisation to 1,092 intravascular imaging-guided PCI (800 with IVUS and 278 with OCT) vs 547 angiography-guided PCI | TVF at a median of 2.1 years |
| ILUMIEN IV (2023)31 | 2,487 patients with medically treated diabetes or complex coronary artery lesions, 1:1 randomisation to OCT-guided PCI (n=1,233) vs angiography-guided PCI (n=1,254) | Post-PCI MSA assessed by OCTTVF at 2 years |
| OCTOBER (2023)30 | 1,201 patients with complex true bifurcation lesions involving both main (>2.75 mm in size) and side branches (>2.5 mm in size), randomised to OCT-guided PCI (n=600) or angiography-guided PCI (n=601); 111 patients (18.5%) in the OCT-guided PCI group and 116 (19.3%) in the angiography-guided PCI group had left main bifurcation lesions | TLF at 2 years |
| OCTIVUS (2023)33 | 2,008 patients randomised to OCT-guided PCI (n=1,005) vs IVUS-guided PCI (n=1,003) | TVF at 1 year |
| Meta-analyses | ||
| Khan et al (2023)16 | 20 RCTs and 11,698 patients comparing intravascular imaging (IVUS or OCT)-guided vs angiography-guided PCI | Cardiac death, MI, stent thrombosis, TVR, or TLR |
| Kuno et al (2023)17 | 32 RCTs and 22,684 patients comparing imaging-guided PCI or functionally-guided PCI vs angiography-guided PCI | Trial-defined MACE (composite of cardiovascular death, MI, and TLR) |
| Giacoppo et al (2024)18 | 24 RCTs (15,489 patients: IVUS vs angiography, 46.4%, 7,189 patients; OCT vs angiography, 32.1%, 4,976 patients; OCT vs IVUS, 21.4%, 3,324 patients) | The two co-primary outcomes were TLR and MI |
| Stone et al (2024)19 | 15,964 patients from 22 RCTs between 1 March 2010 and 30 August 2023, with a weighted mean follow-up duration of 24.7 months | TLF, defined as the composite of cardiac death, target-vessel MI, or TLR |
| *A summary of key results is provided in Supplementary Table 3. FFR: fractional flow reserve; IVUS: intravascular ultrasound; MACE: major adverse cardiovascular events; MI: myocardial infarction; MSA: minimum stent area; NSTEMI: non-ST-segment elevation myocardial infarction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; RCT: randomised controlled trial; TLF: target lesion failure; TLR: target lesion revascularisation; TVF: target vessel failure; TVR: target vessel revascularisation | ||
OCT-guided PCI: procedural techniques and optimisation criteria
A major barrier to the widespread adoption of OCT is the lack of a standardised approach or specific guidance protocol for its integration into routine PCI practice. OCT systems generally offer integrated software automation, allowing for easy incorporation into the routine PCI workflow. A practical algorithm for OCT-guided PCI optimisation, providing step-by-step guidance before, during, and after PCI, is summarised in Figure 1.
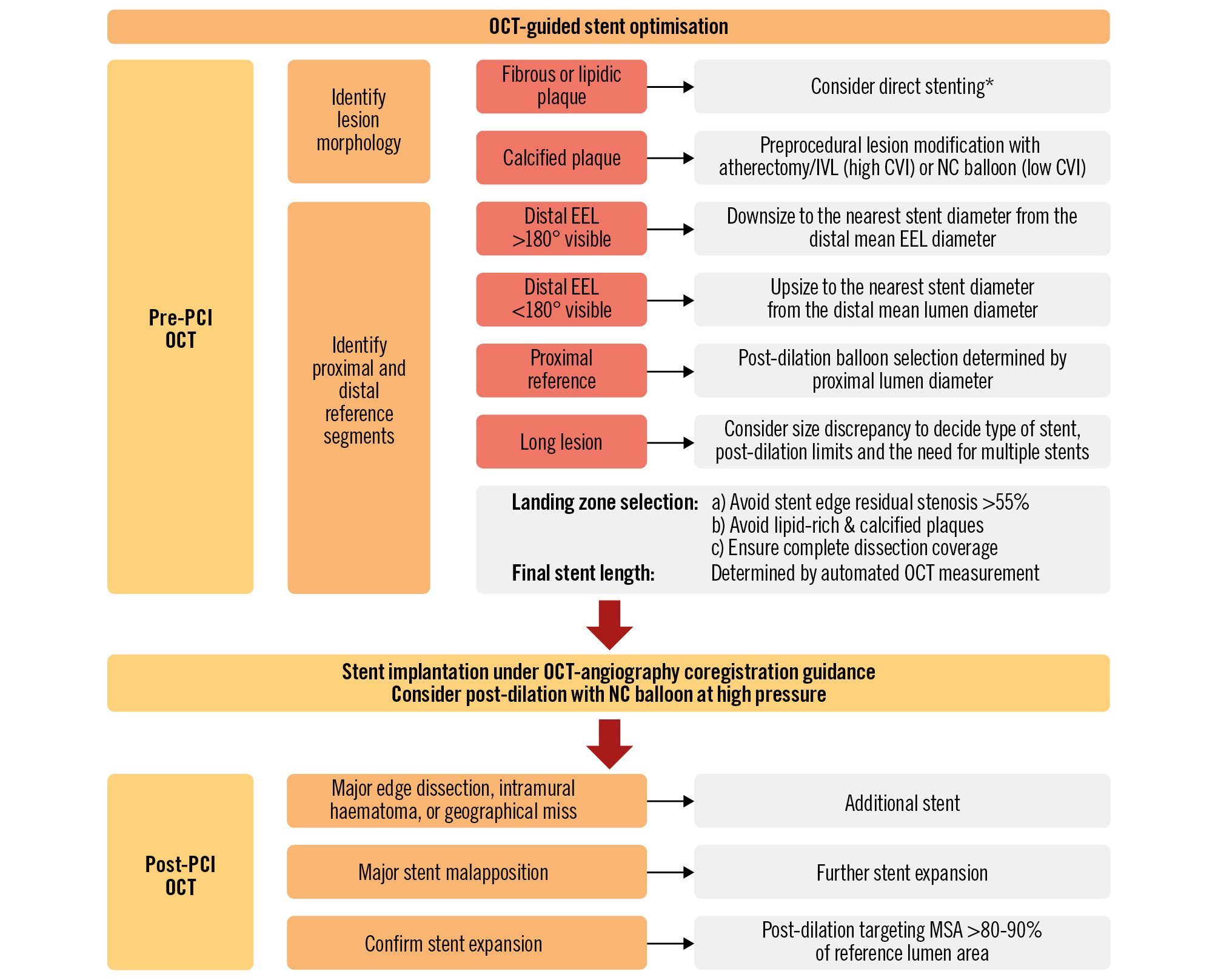
Figure 1. Practical approach for OCT-guided PCI. *While direct stenting might be feasible in the absence of visible calcium, the presence of undilatable or dense fibrous stenosis requires careful evaluation and may necessitate lesion modification prior to stenting. CVI: calcium volume index; EEL: external elastic lamina; IVL: intravascular lithotripsy; IVUS: intravascular ultrasound; MSA: minimum stent area; NC: non-compliant; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
PRE-PCI GUIDANCE
OCT is more accurate in characterising the various components of an atherosclerotic plaque (specifically calcium), and its imaging is helpful to determine the best methods of lesion preparation23536. Fibrous and lipid-rich plaque lesions are frequently treated with a direct stenting strategy or a conventional balloon. By contrast, calcified lesions on OCT may require more aggressive lesion preparation with adjunctive devices. Mild-to-moderate calcification detected by OCT can be managed with non-compliant balloons. However, in cases of severe calcification, specific debulking or lesion modification technologies (e.g., speciality balloons, rotational atherectomy, laser or intravascular lithotripsy) are recommended before stenting373839. An OCT-based calcium scoring system, composed of maximum calcium angle, maximum calcium thickness, and calcium length, can help to identify lesions that would benefit from plaque modification prior to stent implantation37. Severely calcified lesions with a calcium score of 4 (calcium deposit with a maximum angle >180°, maximum thickness>0.5 mm, and length >5 mm) are at risk of stent underexpansion and thus require more extensive plaque modification and calcium-fracturing strategies.
In order to select the optimal stent diameter and length, pre-PCI OCT planning is important to identify the optimal landing zone and reference segment. If the external elastic lamina (EEL) is sufficiently visible on both sides across the lumen centre on OCT, the stent diameter should be determined by measuring the distal reference mean EEL diameter; its diameter should be measured and rounded down to the nearest available stent size143540. However, it should be considered that the reference size can be tapered in diffuse long lesions, which can cause a considerable discrepancy from the proximal to the distal segment, and thus the distal reference EEL diameter would be not enough for stent sizing. Therefore, it is important to consider both the proximal and distal reference sizes, aiming to achieve an optimal MSA that is >90% of the reference segment’s smallest EEL diameter or >80-90% of the average reference EEL diameter, to ensure optimal outcomes3241. In cases where the EEL is not sufficiently visible, a lumen-based sizing strategy is recommended using the mean lumen diameter. When using this approach, the stent diameter should be increased by 0.25-0.5 mm or more, depending on the amount of plaque in the reference segment. It is advised to avoid selecting a landing zone in areas of thin-cap fibroatheroma, lipid pools, and eccentric calcium, which is prone to edge dissections1442.
A practical comparison between IVUS-guided and OCT-guided stent sizing is illustrated in Figure 2. Prior studies have suggested the superiority of an EEL-based sizing strategy over a lumen-based approach, finding it non-inferior to IVUS-guided PCI in achieving the final MSA714. Another study revealed that OCT more accurately measures lumen dimensions than IVUS: the area measured by OCT closely matched that of a phantom model, while IVUS tended to overestimate the area43. Consequently, it is common for the MSA measured by IVUS to be larger than that measured by OCT.
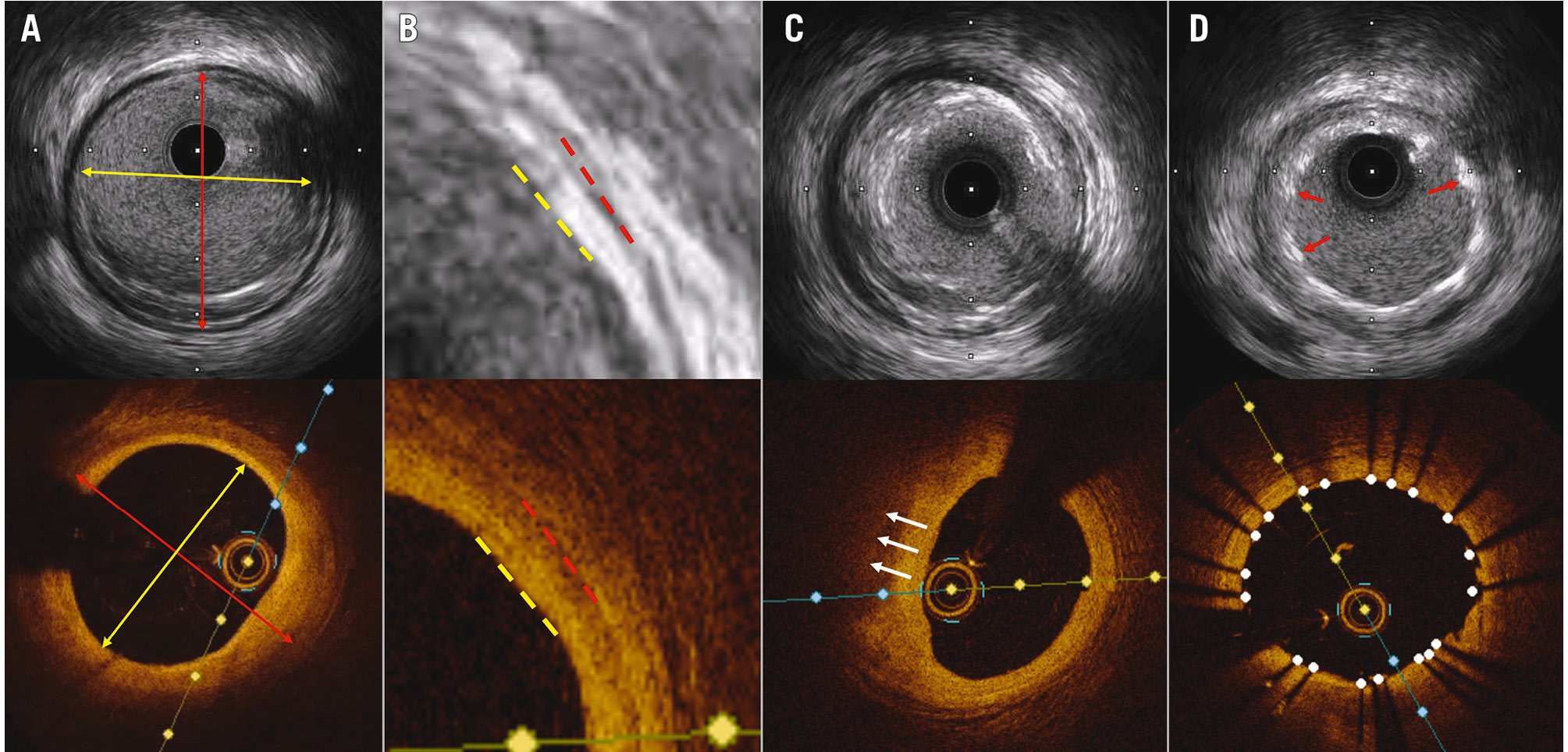
Figure 2. Comparison of IVUS and OCT images in the same coronary lesion. A) IVUS and OCT images of the same coronary lesion, with a lumen area of 7.67 mm2 by IVUS (top), and 7.26 mm2 by OCT (bottom). Red double arrows show EEL diameter measured by IVUS (3.71 mm) and OCT (3.57 mm). Yellow double arrows show lumen diameter (3.16 mm by IVUS and 2.98 mm by OCT). B) Magnified images showing the vessel wall layers with media in IVUS (top, red dashed line), EEL in OCT (bottom, red dashed line), and lumen (yellow dashed lines). C) EEL was visualised by IVUS (top), while plaque attenuation (white arrows) hindered the visualisation of EEL by OCT (bottom). D) Red arrows point at stent struts where the stent area by IVUS was measured as 7.37 mm2 (top). In the OCT image (bottom) with white dots indicating stent struts, the stent area was measured as 7.24 mm2 by OCT. EEL: external elastic lamina; IVUS: intravascular ultrasound; OCT: optical coherence tomography
POST-PCI GUIDANCE
Once the appropriate stent and post-dilation balloon sizes are chosen, post-PCI OCT is employed to evaluate whether additional optimisation processes are required for final PCI optimisation.
Sufficient stent expansion is a critical step in PCI optimisation, as stent underexpansion is strongly correlated with target lesion-related adverse events3674445464748. The most commonly recommended criteria for stent expansion by OCT include (1) an MSA greater than 80% of the mean reference lumen area, and (2) an absolute MSA of more than 4.5 mm2 (for non-left main coronary artery disease)3. The CLI-OPCI registry showed that an OCT-detected MSA >4.5 mm2 was associated with better clinical outcomes49. In the DOCTORS trial7, a target goal for stent expansion was set at 80% of the mean reference lumen diameter, which was found to be comparable to a fractional flow reserve value of>0.90. If stent underexpansion is identified, additional high-pressure balloon inflation should be performed. If stent underexpansion persists after higher-pressure inflation, longer inflations and other advanced strategies (such as ultra-high-pressure balloon inflations, intravascular lithotripsy, and excimer laser) could be considered2.
OCT has a better ability to detect both apparent and subtle stent edge dissections often missed by angiography or IVUS, but the impact of OCT-detected dissections on clinical outcomes is still conflicting4950515253. The CLI-OPCI II study showed that edge dissection >200 μm at the distal stent edge was an independent predictor of worse outcomes49, and another study reported that edge dissection with a flap root thickness>0.31 mm was associated with adverse clinical events54. If there is a major edge dissection (defined as encompassing ≥60° of the vessel circumference and ≥3 mm in length), additional stenting would be required to correct it, unless anatomically prohibitive.
OCT is more reliable than IVUS for the detection of stent malapposition14. Major stent malapposition (defined as unapposed stent struts that are 3 mm long and>0.3 mm from the lumen wall) can be treated using additional high-pressure or semicompliant balloons2. There are conflicting results regarding the relationship between imaging-detected acute malapposition and subsequent coronary events5055565758. Nevertheless, patients presenting with stent thrombosis have commonly identified malapposition as a frequent anatomical abnormality4459. Therefore, extensive malapposition should be avoided after stenting and thus optimally corrected.
With OCT guidance, it is important to check for a geographical miss during a PCI procedure. Residual stenosis and major lipid plaques at the edge of the stented segment detected by OCT could be directly related to the increased risk of TLR; this risk increases with large fibroatheromas and residual stenosis60 and plaque rupture at the edge61. However, the criteria for acceptable residual stenosis vary between studies. Utilising OCT-angiographic coregistration can be instrumental in mitigating geographical miss and in avoiding untreated lipid-rich plaques at stent edges62.
OCT-guided PCI for complex lesions
LEFT MAIN PCI
While earlier OCT models faced technical challenges in imaging the left main coronary artery owing to its large diameter and the difficulty with blood washout, technological advancements with higher acquisition speeds and an expanded field of view have addressed earlier limitations636465. These improvements now enable the proper evaluation and optimisation of stents in the left main coronary artery (representative case in Figure 3). A recent study using frequency-domain (FD)-OCT assessed the technical feasibility of OCT for assessing left main disease66. In this study, FD-OCT was able to accurately evaluate the left main coronary artery and detect and assess angiographically visualised atherosclerotic plaques, providing an accurate assessment of >90% of the quadrants of the left main and the ostia of its daughter branches. Another study showed that FD-OCT assessment of non-ostial left main disease was feasible and provided high-quality imaging64.
In the OCTOBER trial30, the bifurcation lesions sometimes involved the left main coronary artery; this was the case for 18.9% (227 patients) of the total population (111 patients [18.5%] in the OCT-guided PCI group and 116 [19.3%] in the angiography-guided PCI group). In patients with left main bifurcation disease, the 2-year incidence of TLF was lower in the OCT-guided group than in the angiography-guided group (14% vs 19%, respectively, HR 0.79, 95% CI: 0.40-1.51), despite the angiography arm allowing IVUS-guided procedures for left main bifurcation patients. From a clinical viewpoint, the OCTOBER trial confirmed that OCT-guided PCI for left main disease was feasible and contributed to improved PCI outcomes. In a recent comparative analysis of the OCTIVUS trial67, the risk of TVF was comparable between OCT-guided PCI and IVUS-guided PCI for unprotected left main disease.
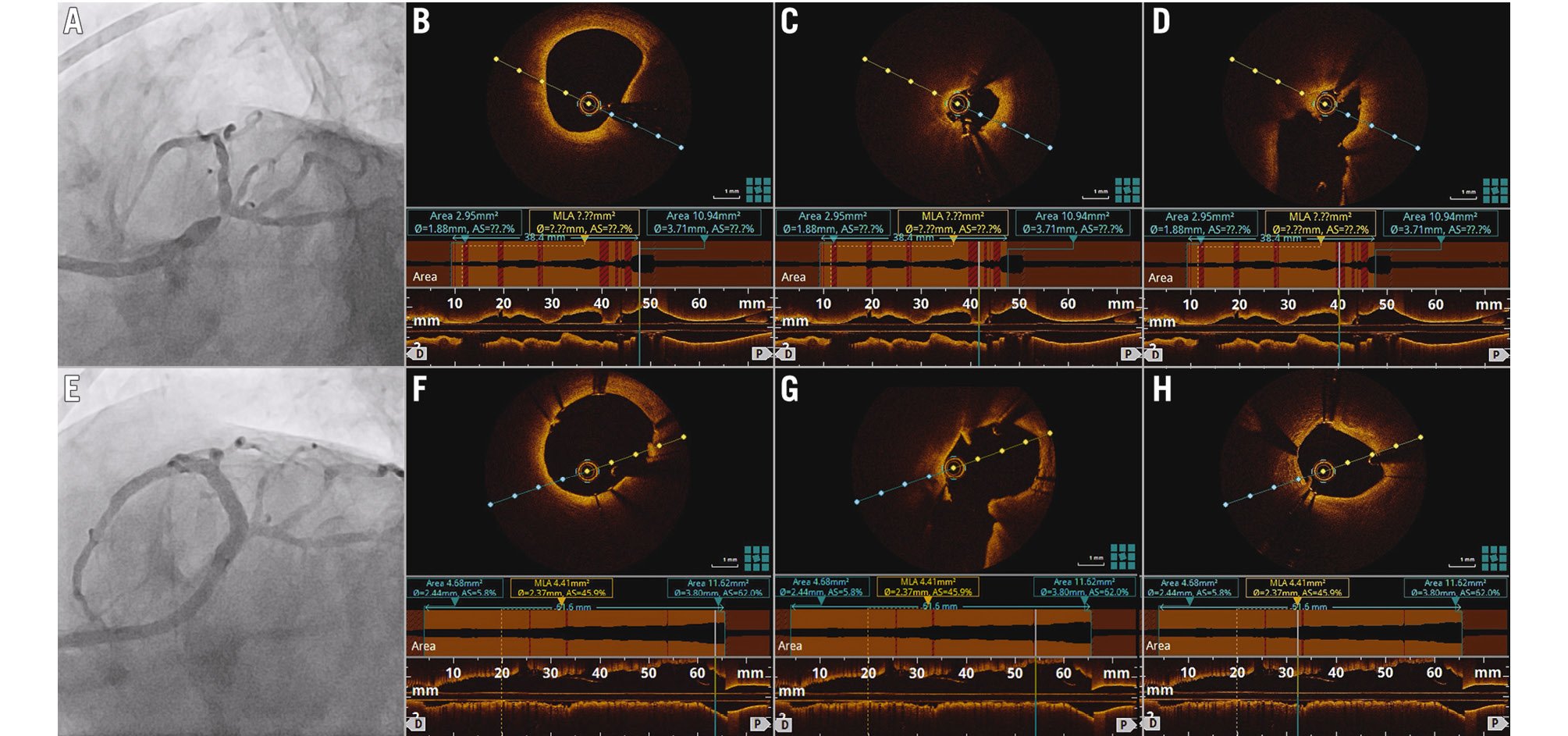
Figure 3. Representative case of OCT-guided PCI for a left main lesion. A) Coronary angiogram showing severe distal left main stenosis. Following predilation with a 2.5 mm non-compliant balloon, OCT imaging showed diffuse stenosis from the distal left main stem to the mid-LAD with a minimal lumen area of 2.95 mm2 (B,C) and a well-opened ostium of the left circumflex, free of disease (D). Two drug-eluting stents of 2.75x38 mm and 2.75x23 mm were implanted in the distal left main to the mid-LAD and dilated with 3.0 mm and 3.75 mm non-compliant balloons. Final angiography and OCT imaging showed a well-apposed proximal stent edge at the left main stem (E,F), well-opened ostium of the left circumflex (G), and an automated minimal stent area measurement of 4.41 mm2 at the distal segment of the mid-LAD (H). LAD: left anterior descending artery; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
BIFURCATION PCI
The OCTOBER trial was the first large-scale RCT to compare OCT-guided PCI with angiography-guided PCI in patients with complex non-left main or left main bifurcation lesions30. Figure 4 shows a representative case of a bifurcation lesion. In patients who were assigned to OCT-guided PCI in the OCTOBER trial, a prespecified, standardised OCT treatment protocol was applied, and the major components of treatment goals on OCT were as follows: (1) optimal lesion coverage (coverage of stenosed segments leaving the 5 mm edge zones adjacent to the stent with <30% stenosis of the reference diameter, absence of major lipid plaque or plaque rupture, and no major edge dissections); (2) optimal expansion (a residual diameter stenosis of the main branch <10%, and the ostium of the side branch showing <50% diameter stenosis with a stent implanted in the main branch only); (3) no malapposition (the absence of stent malapposition was defined as the entire stent having contact with the vessel wall); and (4) no accidentally crushed segment (visual confirmation of no parts of the implanted stent segments being accidentally crushed or distorted). Reference size estimation on OCT was performed with the use of mediaâtoâmedia layer measurement. The reference for each segment was used to select balloons and stents and to evaluate the relative stent expansion after implantation. The 2-year incidence of a primary endpoint event of TLF was significantly lower in the OCT-guided PCI group than in the angiography-guided PCI group (10.1% vs 14.1%, respectively, HR 0.70, 95% CI: 0.50-0.98; p=0.035). The key findings of OCTOBER underscore the particular value of OCT in optimising procedural results for complex bifurcation PCI.
Recently, three-dimensional (3D)-OCT has been incorporated into bifurcation PCI; this technique facilitates the 3D reconstruction of stent geometry, which promotes optimal side branch dilation and reduced stent malapposition or deformation during the procedure23686970717273. Additionally, 3D-OCT offers a clearer visualisation of jailed strut and guidewire positions, which aids in accurate guidewire recrossing and prevent abluminal wiring into the side branch. Technically, distal side branch recrossing with 3D-OCT can minimise incomplete strut apposition and can achieve a wider side branch opening74; while the success rate of optimal distal strut guidewire recrossing is 55-66% with angiographic guidance, it substantially increases to 87-100% with 3D-OCT guidance72.
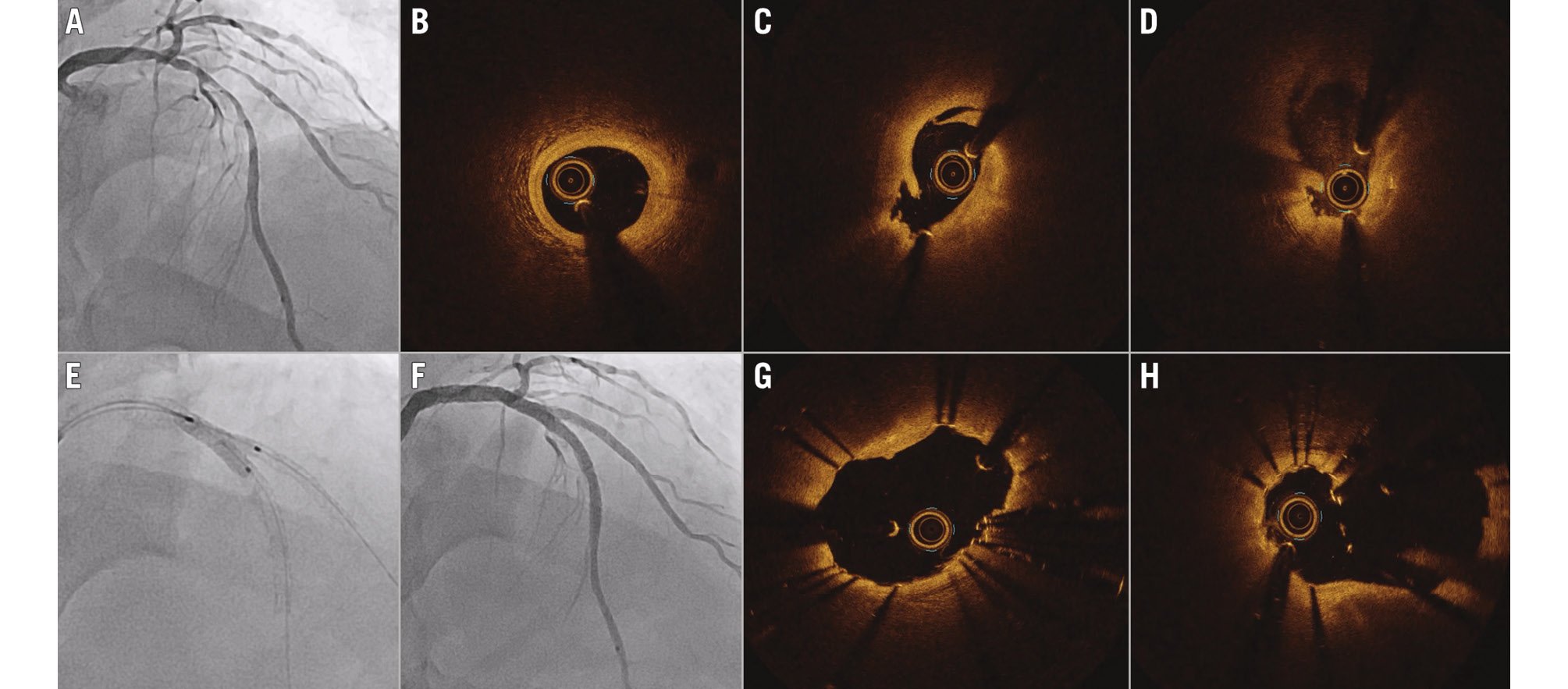
Figure 4. Representative case of OCT-guided PCI for a bifurcation lesion. A) Coronary angiogram showing a Medina 1,1,1 LAD and a diagonal bifurcation lesion. OCT imaging after predilation of the LAD and diagonal branch showed a mild fibrous plaque at the distal reference area (B), fibrofatty plaque with balloon-related dissection at the mid-LAD (C), and ostial dissection of the diagonal branch (D). The bifurcation lesion was treated with a mini-crush technique, in which the diagonal branch was stented with a 2.5x28 mm drug-eluting stent that was later crushed with a 3.0 mm non-compliant balloon parked in the LAD. Overlapping left main to LAD stents that were 3.25x33 mm and 2.75x28 mm were deployed. Sequential high-pressure balloon inflations of the diagonal branch and LAD were followed by a final kissing balloon inflation with 2.5 mm and 3.0 mm non-compliant balloons (E). Final angiography (F) and OCT imaging of the LAD (G) and diagonal branch (H) clearly visualised well-expanded stents with the carina at the bifurcation. LAD: left anterior descending artery; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
CALCIFIED LESION PCI
OCT facilitates the quantitative analysis of calcified lesions (representative case in Figure 5), including measurements of calcium thickness, the angle of the calcium layer, and the length of calcified plaques75. Based on the different types of calcified plaque (deep, superficial, or nodular calcium) detected on OCT, different PCI strategies for optimal lesion preparation and stent optimisation can be applied76. An OCT-based calcium score (based on the calcium volume index) has been developed to predict stent underexpansion and the necessity for prior calcium modification; in cases with a calcium arc greater than 180 degrees, thickness over 0.5 mm and length exceeding 5 mm, adjunctive modification techniques such as rotational or orbital atherectomy, laser angioplasty, or intravascular lithotripsy are strongly recommended37.
The main mechanistic benefits of atherectomy and lithotripsy are plaque fracture and/or abrasion of hard superficial calcium, allowing a more optimised stent expansion. A recent study has revealed that intravascular lithotripsy is highly effective and safe in treating calcified nodules, without major complications77. After modification of a heavily calcified lesion, intravascular lithotripsy can be repeated to confirm calcium fracture before stent implantation. A single-centre Japanese study suggested that OCT-guided rotational atherectomy resulted in greater stent expansion at the calcified target lesion compared to IVUS-guided rotational atherectomy78. However, given the currently limited data from RCTs on the optimal treatment of calcified lesions, further ongoing trials may provide valuable insights for this challenging subset. With the current evidence, it is reasonable to perform OCT to evaluate plaque modification in calcified lesions and to perform further treatment before stenting if dissections are not detected in most severe calcific lesions.
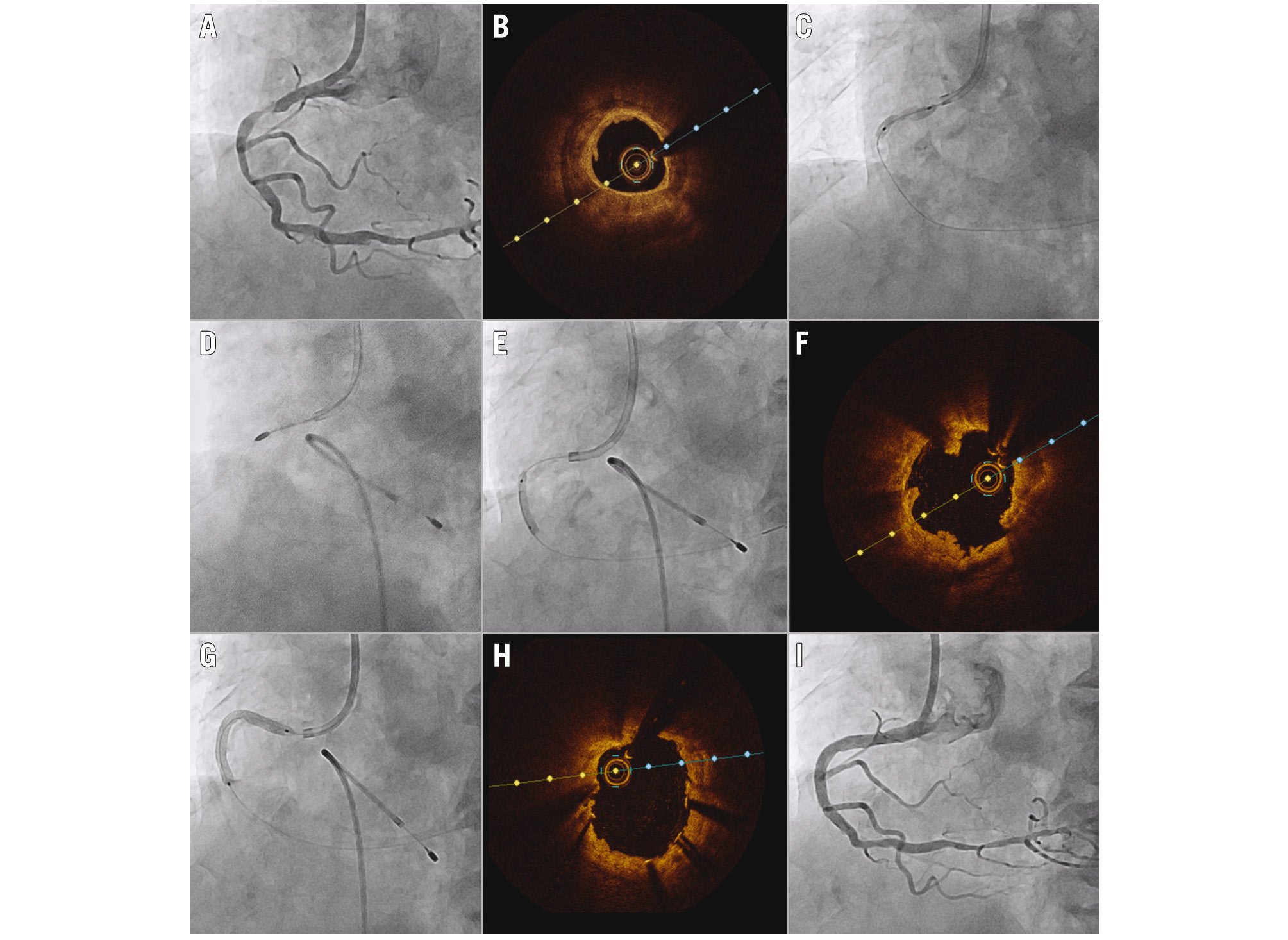
Figure 5. Representative case of OCT-guided PCI for a calcified lesion. A) Coronary angiogram showing a severe stenosis with calcification at the proximal RCA. B) OCT revealed circumferential calcium with >1 mm thickness. C) Predilation with non-compliant and scoring balloons failed to achieve adequate expansion of the lesion. D) A rotational atherectomy with a 1.5 mm burr at 200,000 RPM was performed. E) Further dilation with 2.75 mm and 3.25 mm non-compliant balloons at 24 atm achieved sufficient expansion. F) OCT showed microulcerations and breakage of the calcified plaque. G) A 3.0x38 mm drug-eluting stent was deployed and further dilated with a 3.25 mm non-compliant balloon at 28 atm. Final OCT (H) and angiography (I) showed good stent expansion with an automated minimal stent area measurement of 8.11 mm2. OCT: optical coherence tomography; PCI: percutaneous coronary intervention; RCA: right coronary artery; RPM: rotations per minute
IN-STENT RESTENOSIS PCI
Approximately 4-5% of the overall number of PCIs performed are to treat in-stent restenosis (ISR) lesions7980. Given the limited applicability of coronary angiography in providing information on the underlying mechanisms of ISR, the use of intravascular imaging is helpful for the treatment of ISR (representative case in Figure 6); it can play a significant role in identifying the main causes of stent failure and in deciding the treatment strategy81. Recent clinical guidelines have proposed a Class IIa recommendation (Level of Evidence C) for the use of OCT in determining the mechanism of ISR2029 (Table 3).
In particular, OCT can provide detailed information on distinct entities of ISR mechanisms, such as stent underexpansion, neointimal hyperplasia, and neoatherosclerosis. This information can guide specific treatments: (1) in cases of stent underexpansion, soft tissue is likely to respond to high-pressure balloon inflation, whereas calcified lesions may require adjunctive therapy, such as excimer laser coronary atherectomy, rotational atherectomy, or intravascular lithotripsy82; (2) for neointimal hyperplasia, the use of non-compliant or cutting balloons, followed by drug-coated balloons or an additional drug-eluting stent may be warranted; (3) in cases with multiple layers of ISR, coronary brachytherapy can be a treatment option, as additional stent layers should generally be avoided. Compared to IVUS, OCT has a superior ability to delineate different plaque morphologies and to evaluate the expansion of the original stent and calcium outside the stent; its superior benefit was reported in recent key analyses of the OCTIVUS trial67.
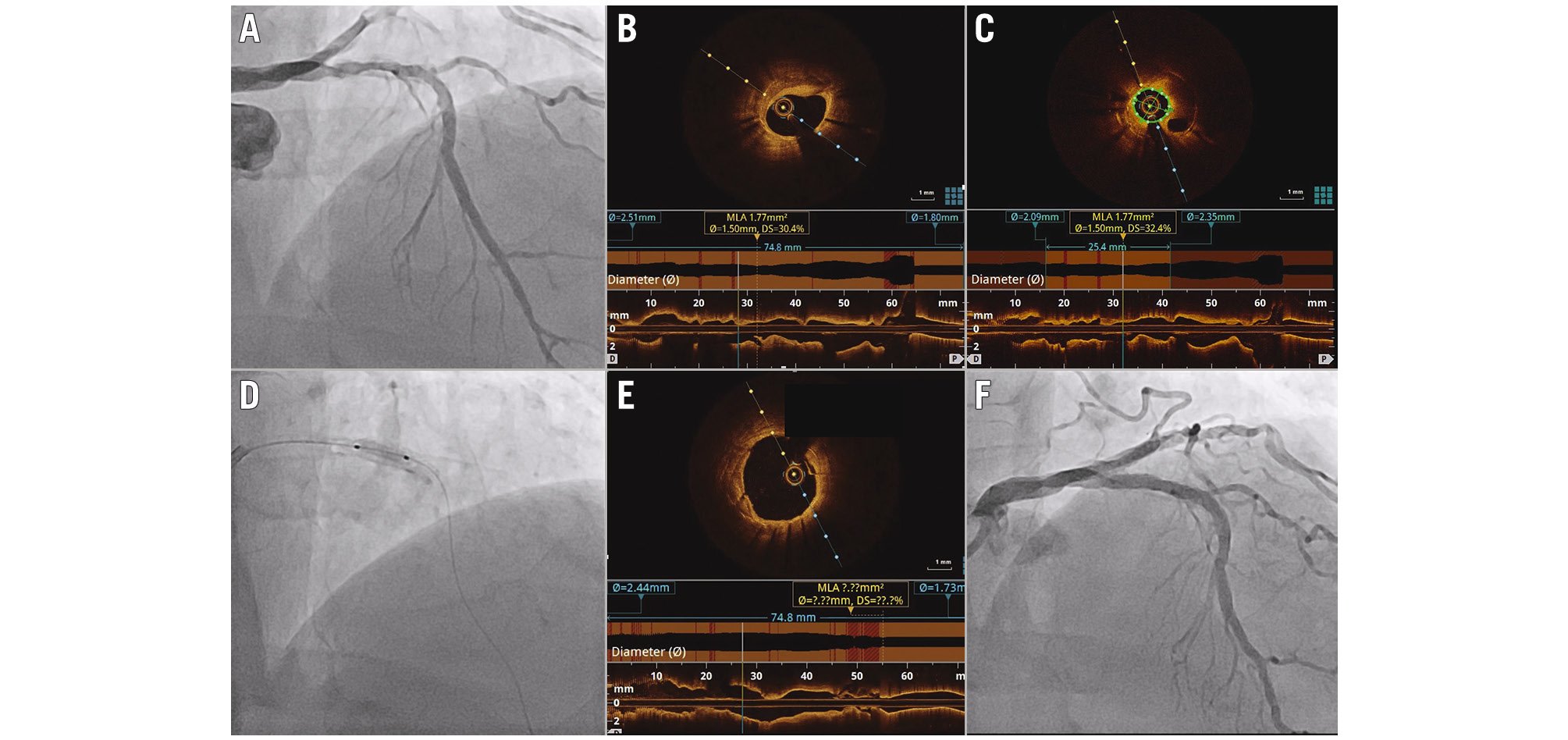
Figure 6. Representative case of OCT-guided PCI for in-stent restenotic lesion. A) Coronary angiogram showing restenosis of the drug-eluting stent that had been implanted in the LAD 13 years earlier. B,C) Baseline OCT revealed calcified neoatherosclerosis with a minimal lumen area of 1.77 mm2. D) Plaque modification with a 3.0 mm cutting and 3.5 mm non-compliant balloon was performed and followed by 60 seconds of inflation of the 3.5 mm paclitaxel-coated balloon. Final OCT (E) and angiography (F) showed sufficient luminal gain with a minimal lumen area of 8.91 mm2. LAD: left anterior descending artery; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Table 3. Contemporary clinical guideline recommendations on imaging-guided PCI.
| Recommendation | Class of Recommendation | Level of Evidence |
|---|---|---|
| Procedural guidance | ||
| 2018 ESC/EACTS29 | IIa: IVUS or OCT should be considered in selected patients to optimise stent implantation | B |
| 2021 ACC/AHA/SCAI20 | IIa: In patients undergoing coronary stent implantation, IVUS can be useful for procedural guidance, particularly in cases of left main or complex coronary artery stenting, to reduce ischaemic events | B |
| IIa: In patients undergoing coronary stent implantation, OCT is a reasonable alternative to IVUS for procedural guidance, except in ostial left main disease | B | |
| Left main stenosis | ||
| 2018 ESC/EACTS29 | IIa: IVUS should be considered to assess the severity of unprotected left main lesions | B |
| IIa: IVUS should be considered to optimise the treatment of unprotected left main lesions | B | |
| 2021 ACC/AHA/SCAI20 | IIa: In patients with intermediate stenosis of the left main artery, IVUS is reasonable to help define lesion severity | B |
| Stent failure | ||
| 2018 ESC/EACTS29 | IIa: IVUS and/or OCT should be considered to detect stent-related mechanical problems leading to restenosis | C |
| 2021 ACC/AHA/SCAI20 | IIa: In patients with stent failure, IVUS or OCT is reasonable to determine the mechanism of stent failure | C |
| Acute coronary syndromes | ||
| 2023 ESC/EACTS29 | IIa: Intravascular imaging should be considered to guide PCI | A |
| IIb: Intravascular imaging (preferably OCT) may be considered in patients with ambiguous culprit lesions | C | |
| ACC/AHA/SCAI: American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions; ESC/EACTS: European Society of Cardiology/European Association for Cardio-Thoracic Surgery; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention | ||
Unmet needs and future perspectives
The evolution of PCI procedures has been significantly influenced by the advent of advanced intracoronary imaging tools. OCT, with its remarkable resolution, stands out as a transformative force in the future PCI landscape, but some technical challenges with OCT use still remain. The mandatory need for blood clearance for OCT image acquisition can increase the amount of radiocontrast used, posing a concern for patients with decreased renal function. While low-molecular-weight dextran and normal saline have been explored as alternatives, they carry their own risks of complications83; thus, the development of more biocompatible and efficient flush media is necessary, which is a key subject of ongoing investigations.
In addition, while full expansion is desirable during imaging-guided PCI, a substantial proportion of imaging-guided procedures fail to meet the expansion criteria – approximately 50% meet the imaging-optimisation criteria3. This suboptimal achievement might often be related to a lack of consideration of vessel tapering and technical challenges in accurately assessing EEL-based stent expansion. The current criteria for imaging optimisation remain arbitrary and lack specificity to individual cases3. This unmet need underscores the importance of further research and technological advancements to enhance the evaluation of multisegment EEL-based stent expansion. Such developments could lead to more uniform and applicable criteria for OCT-guided PCI.
There has been considerable geographical variability in the use of imaging for PCI guidance84. Although a robust body of RCTs confirmed a benefit of imaging-guided PCI, its generalisability and application still remain challenging: hospital culture, a lack of adequate training and education, physician preferences, and costs might hinder the widespread use of intravascular imaging17. The recognition of the benefit of intravascular imaging among healthcare professionals and policymakers as well as the further establishment of affordable reimbursement structures would be essential for widespread acceptance and integration into routine PCI practice.
Anticipated future developments in OCT techniques hold potential for enhanced clinical insights, better accessibility, and ease of use. Since OCT-guided PCI still depends largely on the treating operator’s interpretation and reaction to the imaging findings, considerable interphysician variability may exist. The integration of artificial intelligence offers further promising avenues, particularly in automating image interpretation and streamlining in-lab workflows85. Moreover, the development of multimodality intracoronary imaging systems, merging different imaging methods, promises to revolutionise lesion assessments and PCI optimisation. Also, the combined imaging-physiology assessment (e.g., OCT or IVUS-derived fractional flow reserve) can provide a holistic view of target lesions – a tool that not only offers virtual physiology insights for treatment decision-making but also aids in final PCI optimisation86.
Conclusions
In summary, the trajectory of PCI procedures in the near future is likely to be substantially influenced by a widespread use of intravascular imaging guidance, especially for complex coronary artery lesions. The updated body of contemporary evidence may support a Class I recommendation for the use of intravascular imaging guidance to improve cardiovascular outcomes in patients undergoing PCI. Standardising and simplifying the imaging-guided PCI approach could facilitate the broader integration of intracoronary imaging with OCT or IVUS into routine PCI practice. Moreover, continuous training for practitioners is crucial, and future advancements in technology promise to provide more comprehensive anatomical information and enhance the ease of use. Embracing these innovations will be key to optimising patient outcomes after PCI.
Conflict of interest statement
D.Y. Kang reports speaker fees from Abbott, Daiichi Sankyo, Viatris, Boryoung, and Daewoong Pharmaceutical. S.J. Park reports research grants and speaker fees from Abbott, Medtronic, Daiichi Sankyo, Chong Kun Dang Pharmaceutical, Daewoong Pharmaceutical, and Edwards Lifesciences. D.W. Park reports research grants and speaker fees from Abbott, Medtronic, Daiichi Sankyo, Edwards Lifesciences, Chong Kun Dang Pharmaceutical, and Daewoong Pharmaceutical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

