
Abstract
Myocardial revascularisation represents the most frequently performed therapeutic intervention worldwide. Current percutaneous and surgical revascularisation techniques provide excellent short- and long-term clinical outcomes. However, despite the technological and procedural advances with the widespread use of drug-eluting stents and arterial bypass grafts in contemporary practice, a considerable proportion of patients require repeat revascularisation procedures during long-term follow-up. The need for repeat revascularisation has a major impact on patients’ quality of life and is associated with a significant economic burden. This consensus document summarises the views on the management of myocardial revascularisation failure of an expert panel of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The present document provides a broad and pragmatic overview of the clinical management of myocardial revascularisation failure with a focus on the three key underlying mechanisms leading to repeat revascularisation: 1) failure of percutaneous coronary interventions, 2) failure of coronary artery bypass grafting, and 3) progression of coronary artery disease in native coronary segments previously untreated. The aim of the present position document is to provide a patient-oriented approach for the management of myocardial revascularisation failure.
Introduction
Myocardial revascularisation represents the most frequently performed therapeutic intervention worldwide1,2. Current revascularisation techniques provide excellent clinical outcomes during long-term follow-up1,3. However, approximately 20% of patients undergoing myocardial revascularisation require a repeat revascularisation procedure during the first five years of follow-up, with a higher risk after percutaneous coronary interventions (PCI) as compared with coronary artery bypass grafting (CABG)4,5,6,7. The need for repeat revascularisation has a significant impact on quality of life and healthcare resources, and exposes patients to risks intrinsically related to repeat hospitalisations and invasive procedures4,8,9. Moreover, patients requiring repeat revascularisation are characterised by a high cardiac risk profile, due to comorbidities and anatomical features7,10, rendering their clinical management a significant challenge in daily practice.
This document summarises the views on the management of myocardial revascularisation failure of an expert panel of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). The committee members were proposed by the document chair and co-chair and approved by the EAPCI Scientific Documents and Initiatives Committee.
This document approaches the management of myocardial revascularisation failure from a patient-oriented perspective, based on the underlying mechanisms leading to the clinical need for repeat revascularisation – failure of PCI, failure of CABG, and progression of coronary artery disease (CAD) in native coronary segments previously untreated. The latter is not directly related to overt failure of a previous PCI or CABG. However, from a patient perspective, the need for a new revascularisation procedure represents a failure of the initial treatment strategy and should, therefore, be evaluated in the context of revascularisation failure.
This document has three key objectives: 1) to outline the different mechanisms underlying myocardial revascularisation failure; 2) to detail the specific challenges to the short- and long-term success of repeat revascularisation procedures; and 3) to delineate systematic and informed strategies aimed at increasing the safety and efficacy of these procedures.
Failure of percutaneous coronary interventions
The vast majority of PCI procedures include stent implantation. Stent thrombosis and restenosis are key mechanisms of stent failure requiring repeat revascularisation.
STENT THROMBOSIS
EARLY STENT THROMBOSIS
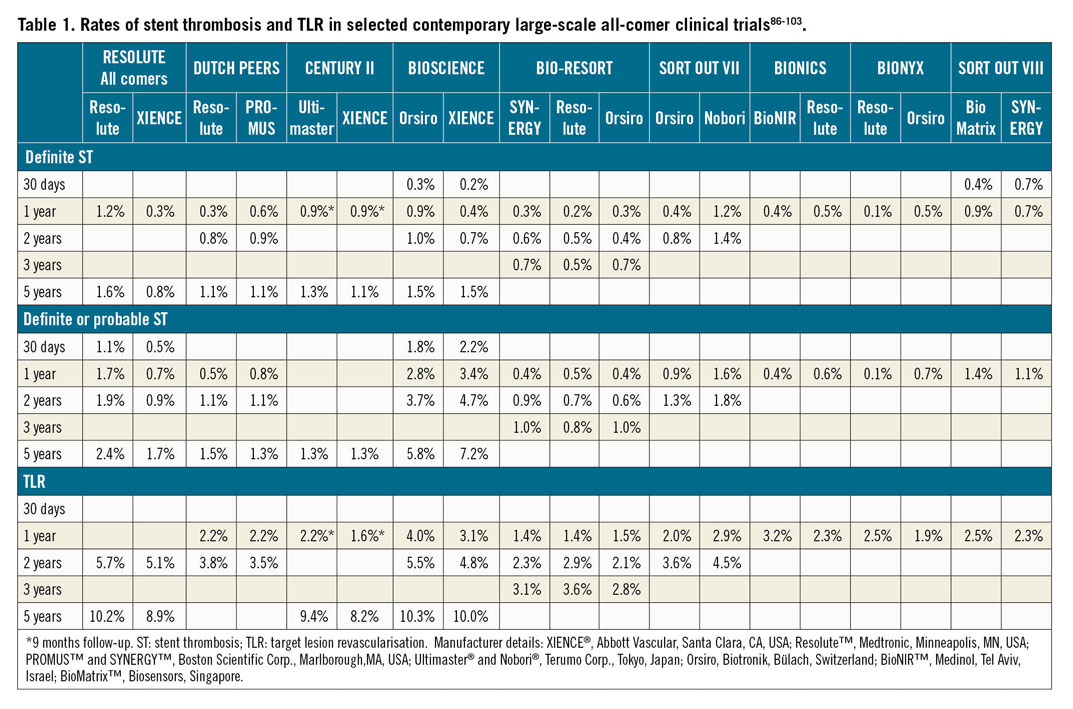
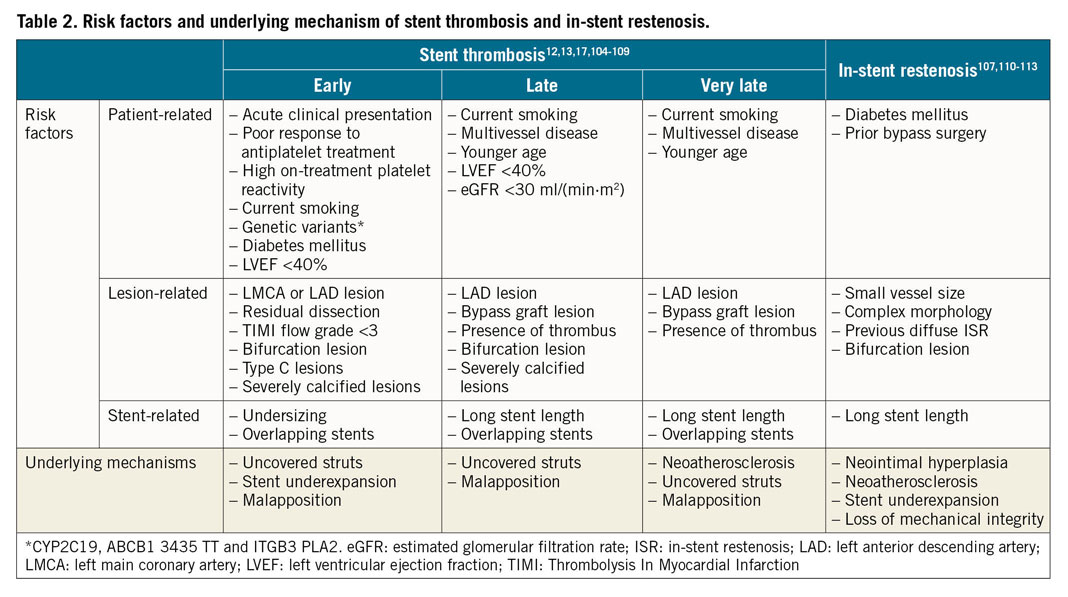
Early stent thrombosis is defined as stent thrombosis occurring within the first 30 days after stent implantation and is subclassified into acute (0-24 hours) and subacute (>24 hours-30 days) stent thrombosis11. Early stent thrombosis is a relatively infrequent occurrence in contemporary clinical practice (Table 1),12. Most cases are related to mechanical or anatomical factors, in association with a thrombogenic milieu or an acute triggering event (Table 2).
LATE AND VERY LATE STENT THROMBOSIS
Late stent thrombosis is defined as stent thrombosis that occurs between 30 days and one year after stent implantation. Very late stent thrombosis is defined as stent thrombosis that occurs later than one year after stent implantation11.
In contemporary large-scale drug-eluting stent (DES) trials with broad inclusion criteria, stent thrombosis rates are low beyond 30 days after stent implantation (Table 1).
The risk factors and underlying mechanisms of late and very late stent thrombosis are summarised in Table 2.
MANAGEMENT OF PATIENTS WITH STENT THROMBOSIS
Most patients with stent thrombosis present with acute myocardial infarction (MI), with or without ST-segment elevation13. Accordingly, the principles of management are those recommended in relevant clinical practice guidelines14,15,16. Usually, patients with suspected ST should undergo urgent coronary angiography to confirm the diagnosis and treat the underlying cause.
Liberal use of intracoronary imaging17 – with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) – is recommended by clinical practice guidelines, in order to detect and modify underlying mechanical factors, and to assess the contribution of concomitant restenosis or neoatherosclerosis to in-stent obstruction14.
In case of a completely occluded vessel, flow should be restored initially, and intravascular imaging should be performed afterwards. In addition to intracoronary imaging, radiological stent enhancement is a helpful method to diagnose loss of stent integrity or underexpansion18. Although routine thrombus aspiration is not recommended by current guidelines, it might be considered in selected cases of stent thrombosis with a large thrombus burden. Similarly, glycoprotein IIb/IIIa receptor antagonists should be considered in view of the elevated prothrombotic milieu. Cangrelor use may be considered in patients not being treated with a P2Y12 inhibitor at the time of stent thrombosis.
Identified factors likely to have contributed to stent thrombosis should be corrected (Figure 1). Patients with deficits in mechanical stent integrity – such as stent gap, stent fracture or longitudinal deformation – as well as those with residual edge disease or dissection should generally be treated with repeat stenting. Stent crush or collapse is very rare, but it may be seen in heavily calcified lesions or at ostial locations; it also mandates repeat stenting. Significant stent underexpansion or malapposition should be corrected with non-compliant balloon dilation, including use of balloons with very high rated burst pressure, as required. Intravascular lithotripsy may be considered for severe, otherwise non-dilatable stent underexpansion19. Following dilation of underexpanded stents, an additional stent may be considered, although systematic repeat stenting in such cases should be avoided, especially if there are already multiple stent layers.
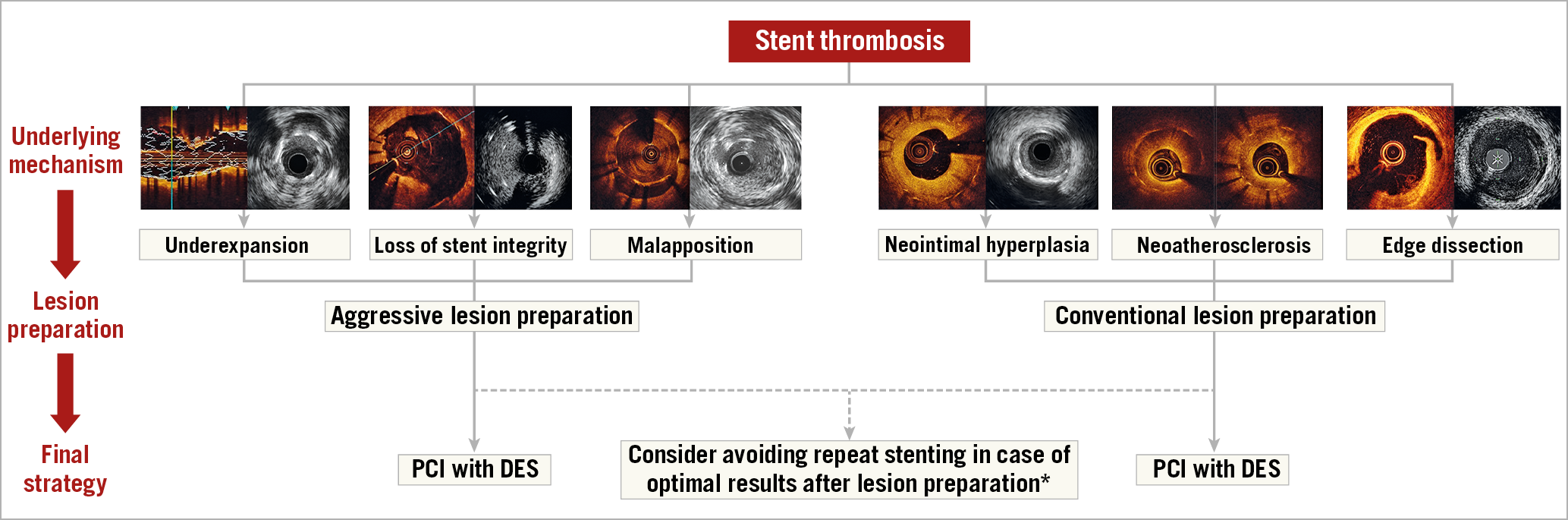
Figure 1. Algorithm for the management of stent thrombosis. *Avoiding stent implantation should be considered in cases with severe underexpansion or malapposition without further underlying mechanisms. In patients with stent thrombosis due to severe neointimal hyperplasia or neoatherosclerosis, PCI with DEB might be considered. Images were kindly provided by Drs Nicolas Amabile, Fernando Alfonso and Gennaro Sardella. DES: drug-eluting stent; PCI: percutaneous coronary intervention
Non-mechanical causes of stent thrombosis may predominate in some cases. These include insufficient platelet inhibition due to hyporesponsiveness, non-compliance to antiplatelet therapy, or interruption for unplanned or non-deferrable surgery. In the absence of clearly identifiable mechanical causes of stent thrombosis, it may be sufficient to dilate the thrombosed stent to restore blood flow and administer antithrombotic agents (e.g., glycoprotein receptor inhibitors, intravenous P2Y12 inhibitors). Subsequently, insufficient platelet inhibition must be evaluated. Use of point-of-care phenotypic and genetic testing has been suggested in patients with stent thrombosis without an evident underlying mechanical cause20,21. Assessment of dual antiplatelet treatment (DAPT) compliance is of paramount importance, especially within the first 30 days after PCI22. Prasugrel and ticagrelor are preferred over clopidogrel after an acute stent thrombosis14. Prolonged DAPT beyond 12 months should be considered in patients after a stent thrombosis, weighing their increased thrombotic risk against their bleeding risk23,24,25.
STENT THROMBOSIS: WHAT TO DO
- Intracoronary imaging with IVUS and/or OCT to identify factors likely to have contributed to stent thrombosis.
- PCI with DES in case of deficits in mechanical stent integrity (stent fracture or collapse).
- PCI with DES in case of residual edge disease or dissection.
- High-pressure non-compliant balloon dilation in case of stent underexpansion or malapposition.
- Assess adherence to antiplatelet therapy.
- Assess platelet reactivity with point-of-care assays in selected cases of acute stent thrombosis without a clearly identified mechanical cause.
- After PCI for stent thrombosis, dual antiplatelet therapy with aspirin 75-100 mg daily and prasugrel 10 mg daily or ticagrelor 90 mg BID for 12 months.
STENT THROMBOSIS: WHAT NOT TO DO
- Systematic repeat stenting in cases of stent underexpansion, especially in the presence of multiple stent layers.
IN-STENT RESTENOSIS
In-stent restenosis (ISR) is a response to vessel wall injury that results in excessive tissue formation (i.e., neointimal hyperplasia or neoatherosclerosis) in the stented segment. ISR is an angiographic diagnosis, defined as a diameter stenosis >50% within the stented segment (i.e., the stent and a 5 mm border proximal or distal to the stent). Although DES were highly effective in reducing the risk of ISR compared with bare metal stents (BMS), ISR remains the most frequent cause of stent failure and the most common indication for target lesion revascularisation (TLR). Large-scale clinical trials of patients treated with contemporary DES with broad inclusion criteria report rates of clinical restenosis (i.e., clinically indicated TLR) of <3% at one year and 10% at five years (Table 1). Of note, ISR presents as an acute coronary syndrome (ACS) in up to 20% of cases26.
Clinical and angiographic factors predisposing to ISR are summarised in Table 2.
MANAGEMENT OF PATIENTS WITH IN-STENT RESTENOSIS
Treatment of ISR is challenging compared with treatment of de novo lesions, owing to relatively high recurrence rates27.
As the underlying substrate in ISR often overlaps with that of stent thrombosis, the principles of management are similar. However, while patients with thrombosis usually present with acute MI, patients with ISR may be asymptomatic and should only be treated in the presence of symptoms or objective evidence of ischaemia. In stable settings, if revascularisation is deemed necessary, the strategy should be carefully planned. As is the case with native coronary artery stenoses, when ISR angiographic severity is unclear, physiological guidance should be considered. If possible, the original lesion and the initial procedure (e.g., material used, maximum balloon pressures, challenges encountered, etc.) should be reviewed to identify potential technical issues that may need to be addressed during the repeat intervention. Intracoronary imaging of restenotic lesions, with IVUS or OCT, may provide insights into the mechanisms underlying ISR (Table 2), by identifying contributing mechanical factors as well as characterising the restenotic tissue type. Of note, in addition to intracoronary imaging, radiological stent enhancement is a helpful method to diagnose stent fracture or underexpansion in patients with ISR18.
There are a number of technical issues that should be considered in the treatment of patients with ISR. Treatment should generally be focused on the stenosed segment rather than on the full length of the stented segment27. To prevent recurrent ISR, it is important to optimise the results of repeat procedures. Careful lesion preparation is required and mechanical issues should be recognised and corrected. Aggressive dilation of the underlying stent might be required, especially in underexpanded or collapsed stents, ideally using non-compliant balloons at high pressures (frequently >18 bar). Care should be taken to avoid geographic miss as this may lead to edge-related recurrence. Use of cutting balloons, or more flexible scoring balloons for lesion predilation, reduces slippage of the balloon out of the stent (so-called “water-melon seeding”), which may lead to stent edge dissections, with the potential for subsequent “candy wrapper” patterns of stent edge restenosis. These devices also incise the surface of the neointimal tissue, which theoretically may facilitate the uptake of drug delivery with drug-coated balloon (DCB) angioplasty or repeat DES implantation. Indeed, the ISAR-DESIRE 4 trial showed improved angiographic outcomes after lesion predilation with a scoring balloon compared with plain balloon angioplasty prior to DCB angioplasty28.
Occlusive ISR constitutes a challenging lesion subset for revascularisation. While the use of a contemporary approach to chronic total occlusion recanalisation is associated with improved procedural outcomes29, long-term results are worse than in de novo chronic total occlusion lesions, largely due to higher TLR rates30.
In the case of resistant stent underexpansion, very high-pressure (25 to 35 bar) balloons may be used. Modification of calcific plaques accounting for stent underexpansion can be performed with excimer laser atherectomy31 or intravascular lithotripsy, the latter also being useful in ISR with calcified neoatherosclerosis32,33. Rotational atherectomy (also termed “rotastenting”) of undilatable underexpanded stents might be considered a second-line strategy but should be undertaken with caution due to the risk of serious complications34. Further study of the therapies discussed is required to confirm their potential benefits.
Following lesion preparation, a proportion of patients will require repeat stenting to correct loss of mechanical integrity of the underlying stent (e.g., due to fracture or gap or, in rare cases, with demonstrated stent collapse). In the remaining patients, after dilatation and correction of any stent underexpansion, a number of treatment options are available, but there is general consensus that additional treatment beyond mechanical dilatation is required as outcomes after plain balloon angioplasty alone are poor35. The two most effective options are DCB angioplasty or repeat stenting with DES36,37. European clinical practice guidelines recommend the use of DES or DCB as first-line therapy in patients with ISR (class I recommendation and level of evidence A for both)14. Repeat stenting with DES seems to be marginally more effective in terms of angiographic recurrences and need for TLR as compared with DCB, particularly in patients with ISR of DES37,38. However, DCB avoid multiple metallic layers on the vessel wall, which may be of particular concern in patients with recurrent ISR. Accordingly, selection between the two strategies may be considered based on the individual characteristics of the patient and lesion to be treated. For instance, DCB may be preferred over DES in ISR of BMS, multiple metal layers, or large side branches. Conversely, DES may be preferred over DCB in lesions with stent fracture, diffuse ISR extending beyond the stent edges, or in case of significant residual dissection or impaired flow after a balloon-only approach (Figure 2). Some operators prefer repeat stenting in the case of ISR at the stent edge, though studies suggest that DCB appear to be as effective for ISR confined to the body of the stent as for those mainly involving its edges39,40.
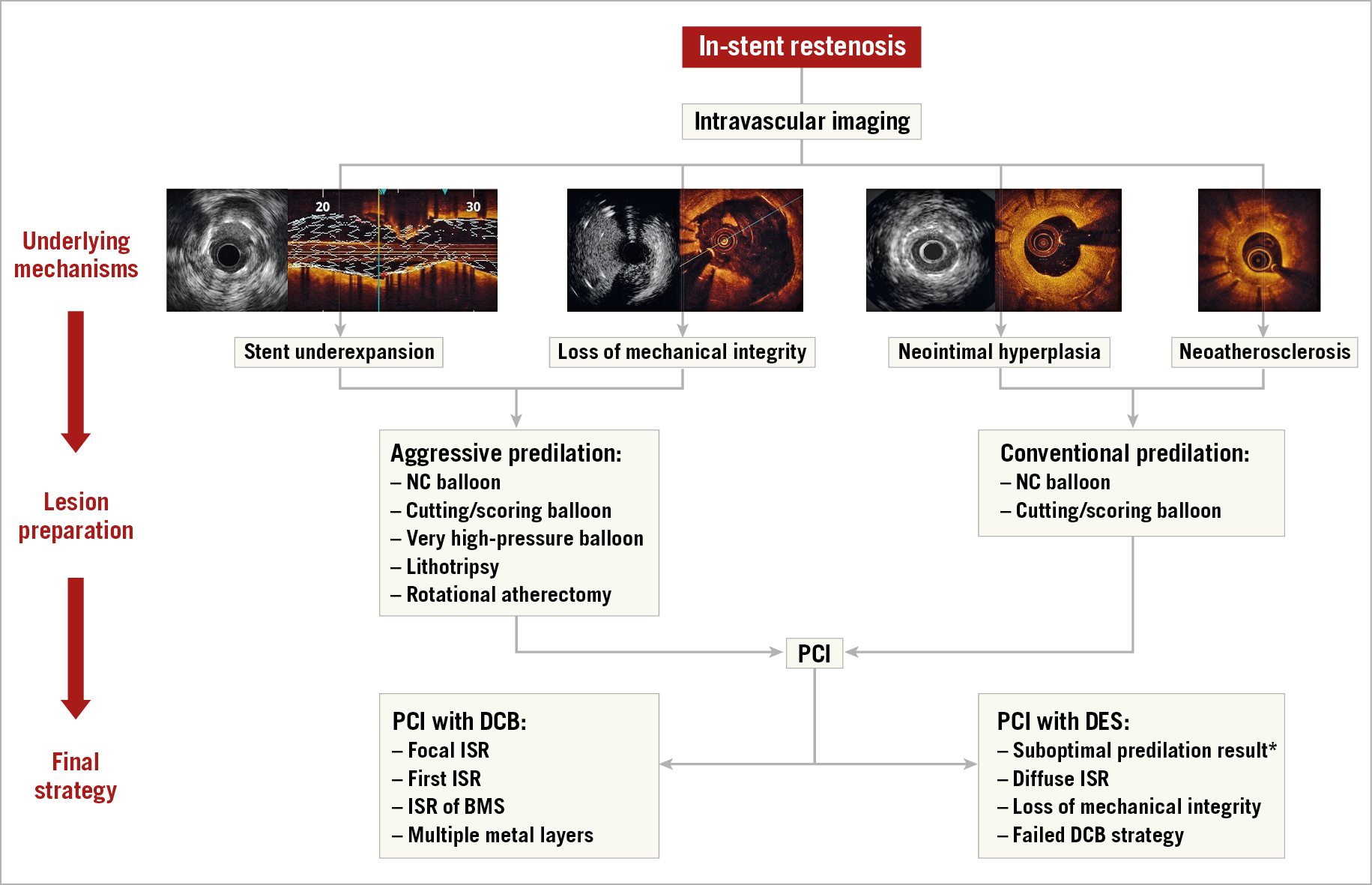
Figure 2. Algorithm for the management of in-stent restenosis. *In patients with edge dissection or acute recoil after lesion predilation, PCI with DES should be considered. Images were kindly provided by Drs Nicolas Amabile, Fernando Alfonso and Gennaro Sardella. BMS: bare metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; ISR: in-stent restenosis; NC: non-compliant; PCI: percutaneous coronary intervention
Antiplatelet treatment for patients undergoing PCI for ISR should not differ from that in patients with a de novo lesion. When ISR clinically presents as chronic coronary syndrome, switching antiplatelet therapy is not recommended unless neoatherosclerosis with plaque rupture or erosion is identified by intracoronary imaging41.
IN-STENT RESTENOSIS: WHAT TO DO
- Intracoronary imaging with IVUS and/or OCT to detect stent-related mechanical problems leading to ISR.
- Aggressive predilation of the underlying stent with non-compliant balloons at high pressure, especially in underexpanded or collapsed stents.
- Lesion preparation with cutting balloons or scoring balloons in order to reduce balloon slippage outside the stent.
- Very high-pressure balloons, intravascular lithotripsy, excimer laser or rotational atherectomy in case of resistant stent underexpansion.
- After adequate lesion preparation, PCI with DES or DCB.
- DES preferred for suboptimal predilation results (residual stenosis >50%, large [large dissection defined if: longitudinal extension >2 mm, lateral extension >60º and involvement of medial or adventitia layers [42]] or flow-limiting dissections), diffuse ISR, loss of mechanical integrity, and failed DCB strategy.
- DCB preferred for focal ISR, first ISR episode, ISR of BMS, and multiple metal layers.
- CABG or a conservative strategy instead of a new PCI attempt in patients with recurrent episodes of diffuse ISR, after a Heart Team discussion.
- After PCI for ACS due to underlying ISR, dual antiplatelet therapy with aspirin 75-100 mg daily and prasugrel 10 mg daily or ticagrelor 90 mg BID for 12 months.
IN-STENT RESTENOSIS: WHAT NOT TO DO
- Treatment of the full length of the initial stent instead of focusing on the stenosed segment.
- Plain balloon angioplasty-only strategy.
ACUTE FUNCTIONAL FAILURE AFTER PCI
Failing to identify haemodynamically significant coronary stenoses is one of the most common reasons portending revascularisation failure. Complementing coronary angiography with invasive functional assessment has received the highest level of recommendation by current guidelines to evaluate the haemodynamic relevance of intermediate-grade stenosis, when non-invasive evidence of ischaemia is not available14. Myocardial revascularisation aims to eliminate ischaemia and is, therefore, expected to normalise findings of invasive functional assessment.
While angiography is considered to have limited ability to assess the haemodynamic relevance of coronary lesions, the adequacy of acute results after PCI is still mainly assessed based on angiographic visual estimation only. However, early evidence with fractional flow reserve (FFR) suggested that suboptimal FFR after stenting is an independent predictor of adverse clinical outcomes at six months43. More recently, a prospective observational study including 574 consecutive patients (664 lesions) with FFR pre and post PCI evaluated clinical outcomes during a mean follow-up of 31±16 months. Despite adequate angiographic result, 143 lesions (21%) had post-PCI FFR values within the ischaemic range (FFR ≤0.80)44.
A meta-analysis that synthesised evidence from 59 observational (prospective and retrospective) studies evaluating the relationship between post-PCI FFR and clinical outcomes found a normal distribution of post-PCI FFR values, with a mean of 0.90±0.04, and indicated that post-PCI FFR values appear to be related to the risk of repeat revascularisation (OR 0.43, 95% CI: 0.34-0.56) and major adverse cardiac events (OR 0.71, 95% CI: 0.59-0.85) during follow-up45. A threshold of final FFR <0.90 has been proposed to define a suboptimal result after stenting46.
Several investigations showed that additional interventions may optimise the acute result in patients with suboptimal post-PCI FFR44,46,47. A recent prospective small-scale study suggested that intracoronary imaging with OCT may reveal potentially treatable causes (i.e., stent underexpansion, incomplete lesion coverage, stent malapposition, edge dissection, or tissue protrusion), allowing optimisation of the post-PCI functional result46. However, whether additional interventions based on post-PCI functional assessment have a significant impact on clinical outcomes has not been clearly determined48.
ACUTE FUNCTIONAL FAILURE: WHAT TO DO
- Repeat invasive functional assessment after stenting when already used to assess the haemodynamic relevance of the treated lesion.
- Attempt to identify reasons for suboptimal (i.e., FFR <0.90) invasive functional assessment post PCI, possibly with the use of intracoronary imaging.
Failure of coronary artery bypass grafting
Surgical graft failure is frequently observed with increasing time after CABG. Graft failure after use of saphenous vein grafts is as high as 50% at 10 years, with vein graft occlusion rates of up to 27% within the first year after CABG49,50,51. Within the first month after surgery, the causes of graft failure are mostly related to the surgical technique and flow pattern-related thrombotic complications, while graft failure thereafter is characterised by neointimal hyperplasia and accelerated progression of CAD52,53,54.
ACUTE GRAFT FAILURE (<1 MONTH AFTER SURGERY)
Acute graft failure can be due to graft dissection, kinking or twisting, anastomotic technical errors, impaired vessel run-off into the native coronary artery, competitive flow from the native coronary artery, or graft thrombosis. In a study of 366 patients with routine post-CABG angiography, 12.2% of the grafts were found to have relevant angiographic defects requiring a minor adjustment of the graft in 2.8%, an anastomosis revision in 3.4%, and intraoperative open-chest PCI in 6.0%52. Because of the logistic issues associated with routine direct postoperative angiography, intraoperative transit-time flow measurements and high-frequency epicardial ultrasound have been used to detect causes of graft failure before chest closure, allowing the opportunity for revision before myocardial ischaemia occurs or progresses.
When clinically relevant, acute graft failure may result in MI with a subsequent risk of mortality. The suspicion of early graft failure should arise in the presence of sudden clinical deterioration as indicated by electrocardiogram (ECG) signs of ischaemia, ventricular arrhythmias, biomarker changes, new wall motion abnormalities, or haemodynamic instability. Due to the low specificity of ECG changes and echocardiographic wall motion abnormalities during the postoperative course and the delay in appearance of biomarker changes, careful assessment of all variables will influence the decision making for angiographic evaluation14.
Despite the fact that arterial grafting is recommended by current guidelines on myocardial revascularisation14, vein grafts continue to be used in larger numbers than arterial grafts, despite having lower long-term patency rates1. Arterial grafts tend to be reserved for the prognostically most important areas of myocardium (e.g., the left internal mammary artery [LIMA] anastomosis to the left anterior descending artery [LAD]). Acute arterial graft failure, therefore, typically has a more severe clinical presentation than vein graft failure, while the latter more often occurs subclinically. An observational study showed that acute graft failure of the LIMA-to-LAD anastomosis warranted reintervention in 80% of patients, while acute vein graft failure was treated conservatively in approximately 50% of patients55.
MANAGEMENT OF ACUTE GRAFT FAILURE
Angiographic assessment is recommended if there is a suspicion of acute graft failure early postoperatively, and is performed in about 1-5% of patients55,56,57,58. In a recent meta-analysis of nine studies and 1,104 patients with suspected perioperative MI after CABG, acute graft failure was diagnosed in 62.1% of patients59. Incomplete revascularisation was the cause of the MI in 6.1% of patients, and 3.5% of patients had a native coronary artery as the culprit. Remarkably, in 31.6% of patients no cause of perioperative MI could be identified. In this context, it is important to underscore that haziness at the anastomosis in this acute period may be difficult to interpret and may not be related to the clinical problem.
The treatment strategy for acute graft failure should be made in an ad hoc Heart Team meeting. As summarised in Figure 3, a number of parameters should be considered in the decision-making process such as the technical reason for acute failure (i.e., problems related with the suture), age and risk profile of the patient, the patient’s clinical condition (e.g., haemodynamic status and inotropic support), pre-CABG native vessel CAD and coronary anatomy, extent and timing of ischaemia, graft configuration, and extent of myocardium at risk.
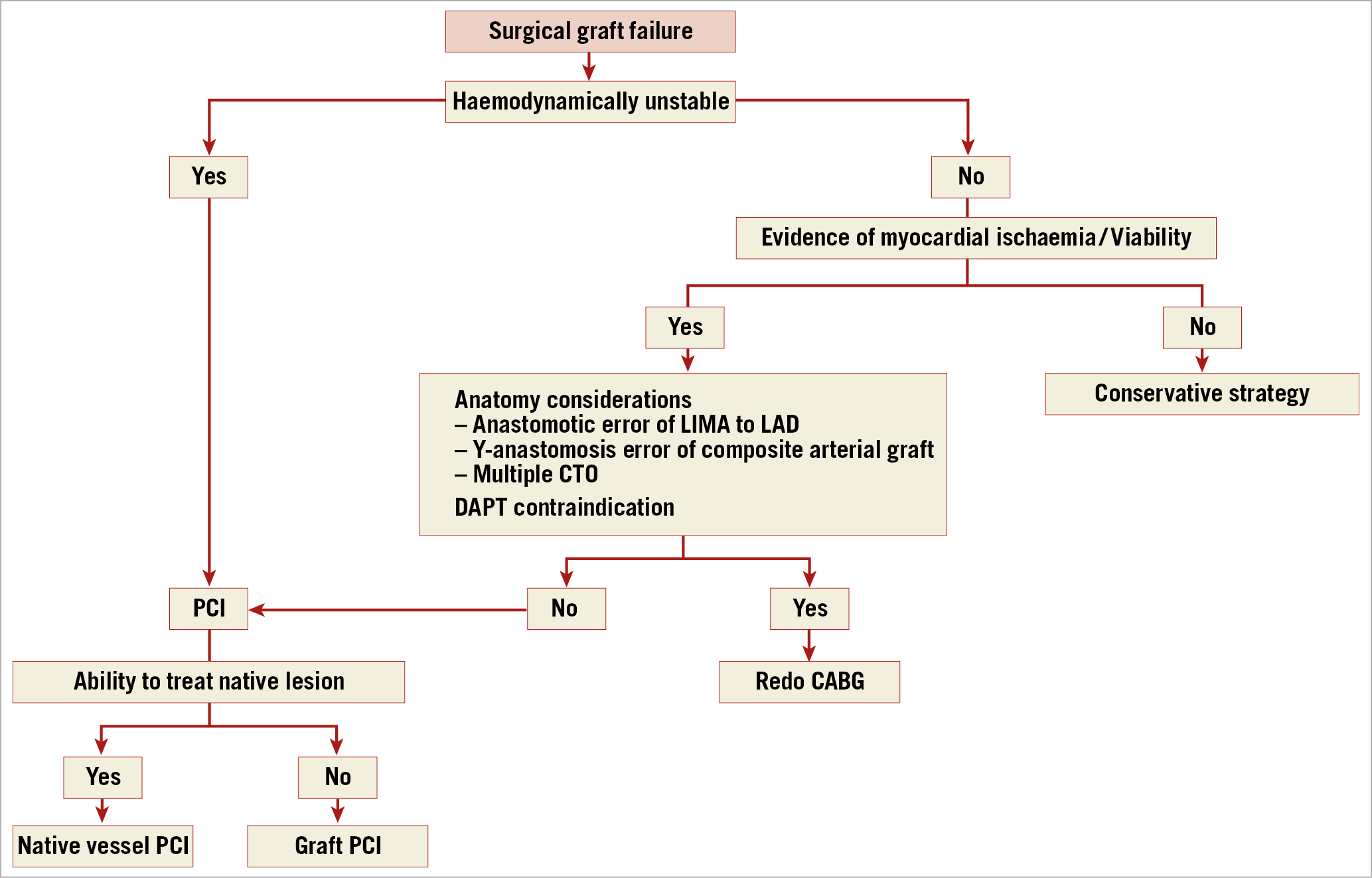
Figure 3. Algorithm for the management of patients with surgical graft failure. CTO: chronic total occlusion; DAPT: dual antiplatelet therapy; LAD: left anterior descending artery; LIMA: left internal mammary artery; PCI: percutaneous coronary intervention
In the setting of acute graft failure, emergency PCI may limit the extent of infarction. Current clinical guidelines advocate that PCI should be the preferred strategy in cases of acute graft failure where the anatomy is suitable14. In such cases, the target for PCI should be the native vessel or the internal mammary artery (IMA) graft, while an acutely occluded vein graft and any anastomotic site should be avoided, if possible, due to concerns regarding fragility of the new anastomosis as well as the risk of embolisation and perforation. The impact of in-hospital PCI following CABG was investigated in a retrospective study in which patients with acute coronary ischaemia requiring PCI after CABG (N=14,323) were compared with those who did not undergo PCI (N=540,664). Post-CABG PCI was associated with an increased risk of unadjusted in-hospital mortality (5.1% vs 2.7%; p<0.001), higher rates of stroke (2.1% vs 1.6%; p<0.001), acute kidney injury (16% vs 12.3%; p<0.001), and a 50% cost increase60.
Redo CABG should be preferred when the anatomy is unsuitable for PCI, when an anastomotic error is evident, or when several important grafts are occluded14. Conservative treatment should be considered in cases where diagnosis has been delayed and viability is expected to be limited. In asymptomatic patients, repeat revascularisation should be considered if the failed graft supplies a large territory of myocardium.
ACUTE GRAFT FAILURE: WHAT TO DO
- Coronary angiography after CABG in patients with sudden clinical deterioration indicated by:
- symptoms of ischaemia and/or abnormal biomarkers suggestive of perioperative MI;
- ischaemic ECG changes indicating a large area of myocardium at risk;
- new significant wall motion abnormalities;
- haemodynamic instability.
- Emergency redo CABG or PCI decided upon by ad hoc consultation in the Heart Team, based on the feasibility of revascularisation, area at risk, comorbidities, and clinical status.
- PCI of the native vessel rather than PCI of the graft.
- Conservative treatment in graft failure cases where diagnosis has been delayed and viability is expected to be limited.
- In asymptomatic patients, repeat revascularisation if the failed graft supplies a large territory of myocardium.
- ACUTE GRAFT FAILURE: WHAT NOT TO DO
- PCI in case of unsuitable anatomy, anastomotic error of the LIMA to LAD or at the Y-anastomosis of a composite arterial graft.
LATE GRAFT FAILURE (>1 MONTH AFTER CABG)
As the time from surgery increases, vein grafts become prone to a process of aggressive and accelerated atherosclerosis. This results in mostly diffuse soft lipid-rich atherosclerotic plaques with extensive necrotic cores with or without intraplaque haemorrhage prone to rupture and downstream embolisation53,54.
Clinically relevant late graft failure presents mostly in the form of stable or unstable angina pectoris61,62,63,64.
MANAGEMENT OF LATE GRAFT FAILURE
A number of critical issues should be considered when treating patients with degenerated grafts, including whether to perform redo CABG or PCI, whether to treat native arteries or degenerated grafts, and the risk of distal embolisation in case of graft intervention.
PCI is considered the treatment of choice in case of late graft failure. Randomised comparisons between redo CABG and PCI, however, are lacking, partly due to patients’ unwillingness to be allocated to redo CABG65. In a subgroup analysis of patients with late graft failure from the AWESOME trial and registry, redo CABG surgery was associated with higher periprocedural mortality as compared with PCI65. Therefore, redo CABG surgery is recommended only in case of extensive native CAD with multiple graft occlusion, particularly in the absence of patent arterial grafts14.
PCI of vein grafts is considered a high-risk intervention due to an increased risk of slow/no-reflow related to distal embolisation of the friable atheroma, depending on the degree of graft degeneration66,67. Embolic protection devices have been proposed to prevent distal embolisation68,69. A randomised trial performed in early 2000 showed a significant benefit of embolic protection devices in PCI of vein grafts68. A similar trend was seen in a subsequent randomised trial that was underpowered due to premature termination69. However, a meta-analysis of 52,893 patients enrolled in these randomised trials and in more recent observational studies did not suggest a benefit of routine use of embolic protection devices in PCI of vein grafts70.
Several randomised trials have compared DES with BMS in vein graft lesions64. In a meta-analysis of randomised evidence, no differences between DES and BMS were observed in terms of all-cause death (RR 1.06, 95% CI: 0.76-1.48), MI (RR 0.81, 95% CI: 0.50-1.29), target vessel revascularisation (RR 0.73, 95% CI: 0.48-1.11) and TLR (RR 1.05, 95% CI: 0.76-1.43) at longest follow-up64. In the ISAR-CABG trial, DES use was associated with a significantly lower risk of TLR during the first year of follow-up (HR 0.49, 95% CI: 0.28-0.86) which was offset by a higher risk between one and five years (HR 2.10, 95% CI: 1.37-3.22) as compared to BMS, with a significant interaction between treatment effect and time (pinteraction <0.001)62,63.
PCI of vein grafts is associated with a higher risk of adverse events as compared to PCI of native coronary arteries among patients with late graft failure71. In a registry of 11,118 veterans, PCI of vein grafts was associated with a significantly higher risk of mortality (adjusted HR 1.30, 95% CI: 1.18-1.42), MI (adjusted HR 1.61, 95% CI: 1.43-1.82) and repeat revascularisation (adjusted HR 1.69, 95% CI: 1.50-1.71) as compared to PCI of native arteries during a median follow-up of three years71.
Although available evidence clearly supports PCI of the native artery in case of late graft failure, anatomical complexities – such as multiple chronic total occlusions of native arteries – might limit the success of such a strategy, forcing interventionalists to treat degenerated grafts instead. Despite improvements in recanalisation techniques and available dedicated tools, previous CABG surgery remains one of the most important predictors of PCI failure in chronic total occlusions72. Therefore, the decision to treat native artery lesions or surgical grafts depends on CAD anatomical complexity and the interventionalists’ expertise in complex PCI, seeking the most complete revascularisation. The decision should be made on an individual patient basis, giving priority to PCI of native arteries.
LATE GRAFT FAILURE: WHAT TO DO
- PCI as first choice over redo CABG for late graft failure.
- PCI of the native vessel rather than PCI of the graft.
- PCI strategy based on operator experience in complex PCI.
- Distal protection devices for PCI of vein graft lesions with diffused degeneration.
- IMA for redo CABG in patients in whom the IMA was not used previously.
- Redo CABG in patients without a patent IMA graft to the LAD, after checking its patency.
- Redo CABG in case of extensive native CAD, anatomically unsuitable for PCI, in the absence of patent grafts (especially arterial).
LATE GRAFT FAILURE: WHAT NOT TO DO
- Routine use of embolic protection devices for PCI of vein grafts.
- Plain balloon only for PCI of the graft.
REPEAT REVASCULARISATION DUE TO PROGRESSION OF CAD
CAD progression in native coronary segments previously untreated is the primary cause of repeat procedures after myocardial revascularisation.
NATIVE CAD PROGRESSION AFTER PCI
Disease progression is responsible for a relevant proportion of repeat revascularisation procedures after PCI6, although the incidence varies based on the clinical and anatomic characteristics of the population studied. The Prospective Natural History Study of Coronary Atherosclerosis (PROSPECT) studied the relative contribution of events related to the initially treated lesion (culprit lesion) and events related to CAD progression in non-culprit sites among 697 patients with ACS undergoing PCI73. The cumulative rate of major adverse cardiac events – a composite of cardiac death, arrest, MI, and hospitalisation for angina – was 20.4% at three years, with 12.9% of events related to the culprit lesion and 11.6% of events due to CAD progression at non-culprit sites. Overall, 65% of all events occurred within one year after PCI, with a relatively equal distribution between events related to the culprit lesion and those related to CAD progression. The overall repeat revascularisation rate was 17.1% at three years, with an equal contribution of events related to the culprit lesion and those related to CAD progression.
Predictors of CAD progression in previously untreated native coronary segments include clinical and angiographic factors that are largely overlapping with predictors of PCI and CABG failure, such as age, diabetes mellitus, complex coronary anatomy, extent of CAD, small vessel CAD, and previous PCI of vein grafts or ostial lesions6,74.
NATIVE CAD PROGRESSION AFTER CABG
Current recommendations for CABG inherently select patients at higher risk for native CAD progression. These include patients with multivessel CAD, high anatomical complexity and extent of CAD, and coexistence of multiple comorbidities including diabetes mellitus, reduced left ventricular ejection fraction, and chronic kidney disease. Historical evidence indicates that accelerated CAD progression occurs up to tenfold more frequently in non-obstructive atherosclerotic lesions in bypassed coronary arteries compared with similar lesions in non-bypassed vessels at three years after CABG75. In another study, the risk of CAD progression was twice as high in arteries with patent grafts as compared to those with closed grafts, with the majority of grafted arteries with CAD progression being completely occluded. A more recent analysis of contemporary surgical techniques showed similar results, with development of a new chronic total occlusion in a native coronary artery in >40% of patients within one year after CABG, strongly predicted by a severe (>90%) proximal stenosis in the same vessel76.
GENERAL PRINCIPLES FOR MANAGEMENT OF CAD PROGRESSION
In case of CAD progression in previously untreated native coronary segments following revascularisation, treatment recommendations should be based on symptoms and evidence of myocardial ischaemia. In this context, optimal medical therapy plays a pivotal role not only to reduce the risk of CAD progression but also for an initial management of patients with evidence of CAD progression. We refer to relevant clinical practice guidelines for a comprehensive assessment on recommendations for optimal medical management, which represents the cornerstone for prevention and treatment of CAD progression14,77,78. The interventional management of CAD progression differs according to the initial revascularisation modality.
MANAGEMENT OF CAD PROGRESSION AFTER PCI
In contemporary large-scale PCI trials, up to one third of patients enrolled were previously treated with PCI79,80,81. Percutaneous treatment of CAD progression after a previous PCI is generally reasonable. A surgical revascularisation strategy may be appropriate in case of CAD progression involving proximal segments of major coronary arteries or multivessel disease involving the left main or proximal LAD. A large registry that evaluated outcomes of patients with previous PCI undergoing CABG showed that early mortality and adverse ischaemic events did not significantly increase in patients with single or multiple previous PCI procedures82. Therefore, a strategy based on clinical and anatomical factors similar to that for patients with a first diagnosis of CAD is recommended in patients with CAD progression after PCI (Figure 4).
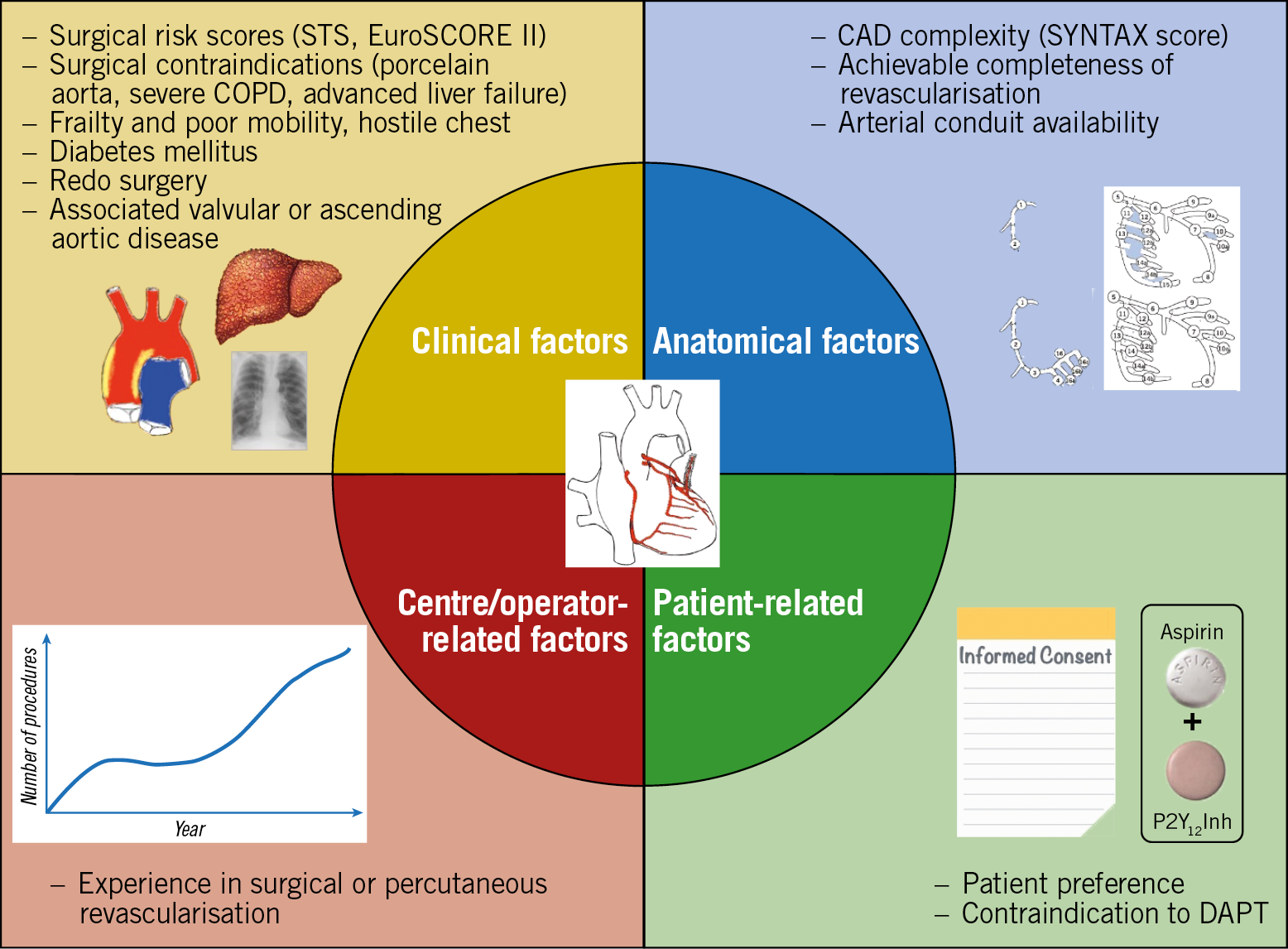
Figure 4. Factors that may guide revascularisation strategy for CAD progression. CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DAPT: dual antiplatelet therapy; STS: Society of Thoracic Surgeons
MANAGEMENT OF CAD PROGRESSION AFTER CABG
Repeat revascularisation procedures after CABG are typically performed in older patients with more comorbidities and more complex coronary anatomy as compared to patients with a first diagnosis of CAD. Furthermore, in these patients arterial conduits tend to be less frequently available, having already been used83. Therefore, redo CABG is associated with increased procedural risks and worse clinical outcomes compared with a first CABG. Recent evidence indicates a trend towards a decreased risk of adverse events in patients treated with PCI coupled with an increase in PCI use in this setting66,84. In view of the paucity of available comparative effectiveness evidence, in these patients the selection of the repeat revascularisation strategy should be based on the assessment of clinical and anatomical risk profiles on an individual patient basis in discussion within the Heart Team (Figure 3, Figure 4),65,85.
CAD PROGRESSION: WHAT TO DO
- Repeat revascularisation in patients with evidence of CAD progression and with a large area of ischaemia or severe symptoms despite medical therapy.
- Base the selection of the repeat revascularisation strategy on the assessment of clinical and anatomical risk profiles on an individual basis in the context of the Heart Team.
- If considered safe, PCI with DES as first choice over CABG.
- IMA for redo CABG in patients in whom the IMA was not used previously.
- Redo CABG in patients without a patent IMA graft to the LAD.
CAD PROGRESSION: WHAT NOT TO DO
- Routine invasive angiography tests in asymptomatic patients with prior revascularisation.
- Routine ad hoc PCI in patients with progression of CAD after CABG.
Conclusions
Current percutaneous and surgical revascularisation techniques are associated with excellent procedural and long-term clinical outcomes. However, a considerable proportion of patients require repeat revascularisation procedures during long-term follow-up due to failure of the initial revascularisation – either PCI or CABG – or progression of disease in previously untreated coronary segments. This document provides evidence-based guidance for the management of myocardial revascularisation failure based on the underlying mechanism, the timing and the clinical and angiographic characteristics of individual patients.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
G.G. Stefanini reports a research grant (to the institution) from Boston Scientific and speaker/consulting fees from B. Braun, Biosensors, and Boston Scientific. R.A. Byrne reports lecture fees from B. Braun Melsungen AG and Biotronik, and research funding to the institution of employment from Celonova Biosciences. D. Capodanno declares consulting honoraria from Abbott Vascular, Bayer and Daiichi Sankyo, and speaker fees from AstraZeneca, Biosensors, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi Aventis. E. Barbato declares speaker’s fees from BSCI, Abbott Vascular, and GE. V. Kunadian reports consulting fees/honoraria from Bayer, Amgen, Daiichi Sankyo, Abbott Vascular, and AstraZeneca, and a major institutional research grant from AstraZeneca. J. Mehilli reports personal fees from AstraZeneca, has received lecture fees from Abbott Vascular, Boston Scientific, Biotronik, Edwards Lifesciences, Bristol-Myers Squibb, Medtronic and Terumo, and has received institutional research grants from Edwards Lifesciences, Boston Scientific, and Abbott Vascular. D. Regazzoli reports speaker honoraria from Amgen and Boehringer. A. Baumbach reports institutional research support from Abbott Vascular and honoraria from AstraZeneca, Sinomed, MicroPort, Abbott Vascular, Cardinal Health, and KSH. F.J. Neumann reports personal fees from Amgen, Boehringer Ingelheim, and Daiichi Sankyo, grants and personal fees from Pfizer, Biotronik, Edwards Lifesciences, Bayer Healthcare, and Boston Scientific, personal fees from Novartis, grants from Medtronic, and GlaxoSmithKline, and personal fees from Ferrer, outside the submitted work. W. Wijns reports grants and personal fees from MicroPort, outside the submitted work, being a medical advisor to Rede Optimus Research, and being a co-founder of Argonauts, an innovation facilitator. The other authors have no conflicts to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

