
Introduction to the session
The aim of the summary report is to capture the session at EuroPCR 2016 that reviewed the implications for clinical practice of SPRINT (Systolic Blood Pressure Intervention Trial)1, in order to share the critical analysis of the trial and report the views expressed in the interactive discussion. This article does not constitute an independent review of the topic by the authors.
SPRINT certainly addresses a clinically relevant problem in attempting to improve the definition of optimal targets for systolic blood pressure (SBP) lowering in a hypertensive population at varying levels of cardiovascular risk. However, before answering the question whether this trial will change our practice in treating hypertensive individuals, established data and the SPRINT trial methodology should be addressed.
Background: what was known before SPRINT
Hypertension is highly prevalent and is a major cause of morbidity and death worldwide. Projections for the next decades suggest that up to 50% of the adult population will be diagnosed with hypertension using currently defined blood pressure (BP) thresholds. Numerous clinical trials have provided evidence that reducing blood pressure in hypertensives decreases the incidence of stroke, myocardial infarction and heart failure. The current ESH/ESC guidelines recommend treatment with a goal of SBP <140 mmHg in most patients and a less strict target pressure of <150 mmHg in the elderly2. Prior to SPRINT, various randomised controlled trials investigated whether a more intensive target was better than standard control. These included the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial3, which was restricted to patients with diabetes, and the Secondary Prevention of Small Subcortical Strokes (SPS3) trial4, which was restricted to patients with lacunar infarcts. In ACCORD, there was no benefit of lowering SBP to <120 mmHg compared with <140 mmHg in terms of non-fatal stroke or death from cardiovascular causes3. Furthermore, in SPS3, lowering SBP to <130 mmHg compared with <150 mmHg did not change the overall risk of another stroke amongst patients who had had a stroke4. However, the patients in the intensively treated group in this trial had fewer stroke events4. It is noteworthy that both diabetics and patients with prior stroke were excluded from SPRINT. The most recent meta-analysis of intensive BP lowering (prior to the publication of SPRINT) included 19 trials in total with 44,989 participants with a mean duration of 3.8 years of follow-up5. Patients in the more intensive blood pressure-lowering treatment group had a mean BP 133/76 mmHg vs. 140/81 mmHg in the less intensive treatment group, and achieved relative risk reductions for major cardiovascular events (14%), myocardial infarction (13%), stroke (22%), albuminuria (10%), and retinopathy progression (19%). Of note, more intensive treatment did not result in effects on cardiovascular death, total mortality, heart failure or end-stage kidney disease.
In contrast, it is also important to note that, whilst there is a clear and progressive increment in cardiovascular risk with SBP levels greater than 115 mmHg (entered as a time-varying covariate), there is also an opportunity for harm in aiming to reduce BP levels back towards this baseline. In the LIFE study, 9,193 hypertensive patients with left ventricular hypertrophy (LVH) diagnosed by ECG were randomly assigned to losartan or atenolol-based treatment. Univariate analyses demonstrated that, compared with in-treatment SBP >142 mmHg, in-treatment SBP 131-141 mmHg entered as a time-varying covariate identified patients with significantly lower risk of all events6. Of note, patients with SBP 130 mmHg or less had less reduction in MI, stroke and no significant decrease in cardiovascular or all-cause mortality.
Trial analysis: summary of the trialist’s critical review
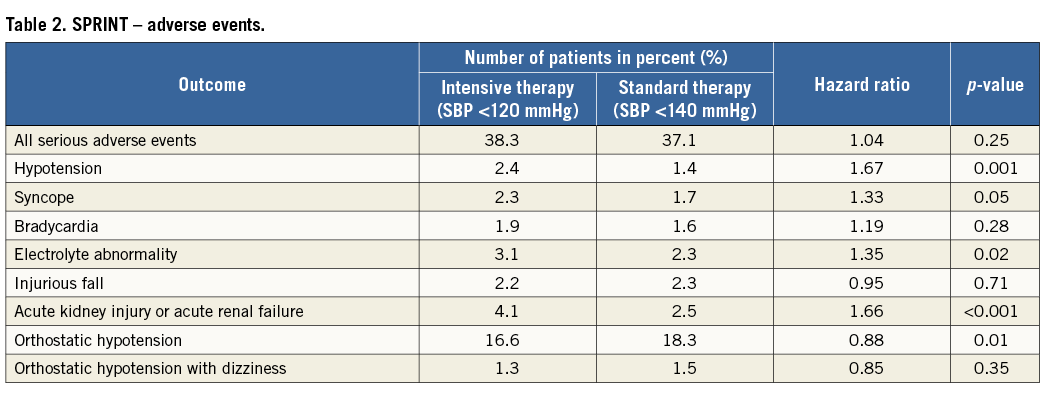
SPRINT was a randomised, controlled, open-label trial sponsored by the National Institutes of Health (Bethesda, MD, USA). The effects of antihypertensive therapy with SBP targets of <120 mmHg (intensive treatment) and <140 mmHg (standard treatment) were compared in 9,361 hypertensive adults aged ≥50 years, with SBP from 130 to 180 mmHg and at least one cardiovascular risk factor, including clinical or subclinical cardiovascular disease (other than stroke), chronic kidney disease, a Framingham risk score of ≥15% or age ≥75 years. Participant enrolment occurred between 2010 and 2013 at 102 sites in the United States and Puerto Rico. Patients with diabetes, prior stroke, polycystic kidney disease, end-stage renal disease or proteinuria (excretion >1 g/day) were excluded. Unattended BP was assessed by an automated manometer (Omron Healthcare, Lake Forest, IL, USA) and the average of three office BP readings was used for analysis. SPRINT was terminated prematurely following the recommendation of the data safety monitoring board. Up until termination, a total of 564 primary endpoint events occurred, providing reassurance that the results were valid. The main finding of SPRINT was that the primary endpoint, consisting of myocardial infarction, acute coronary syndrome, stroke, acute decompensated heart failure and cardiovascular death was reduced by approximately 25% in the intensive treatment group compared with the standard treatment group (Table 1). More specifically, during a mean follow-up period of 3.2 years, intensive BP control prevented 76 events (number needed to treat [NNT] 61). Similarly, all-cause mortality was reduced by approximately 27% in the intensive treatment group (NNT 83) (Figure 1). There were surprisingly high rates of adverse events with a total of 1,793 (38%) serious adverse events occurring in the intensive group compared with 1,736 (37.1%) in the standard group (Table 2). However, hypotension, syncopal episodes, acute kidney injury and serum electrolyte abnormalities (lowered sodium and potassium) were more common in the intensive treatment group. Furthermore, the long-term effect on renal function remains unclear.
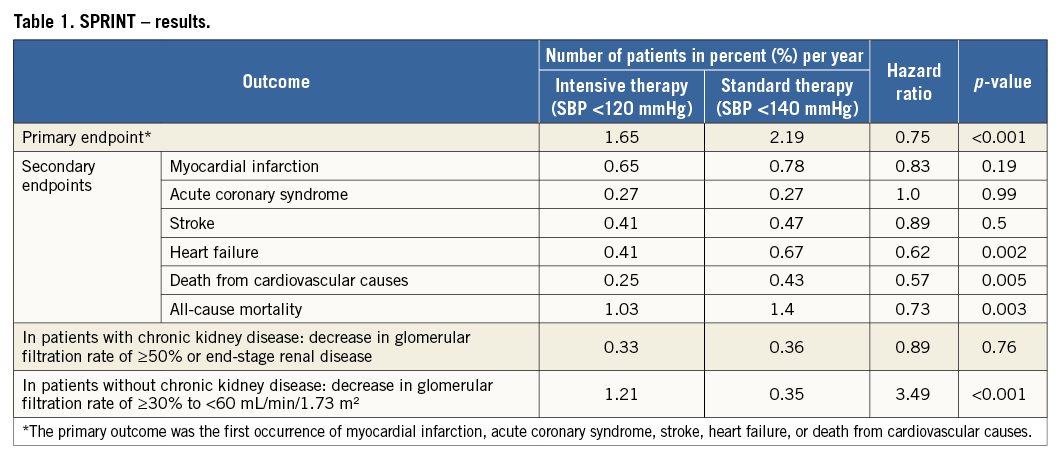
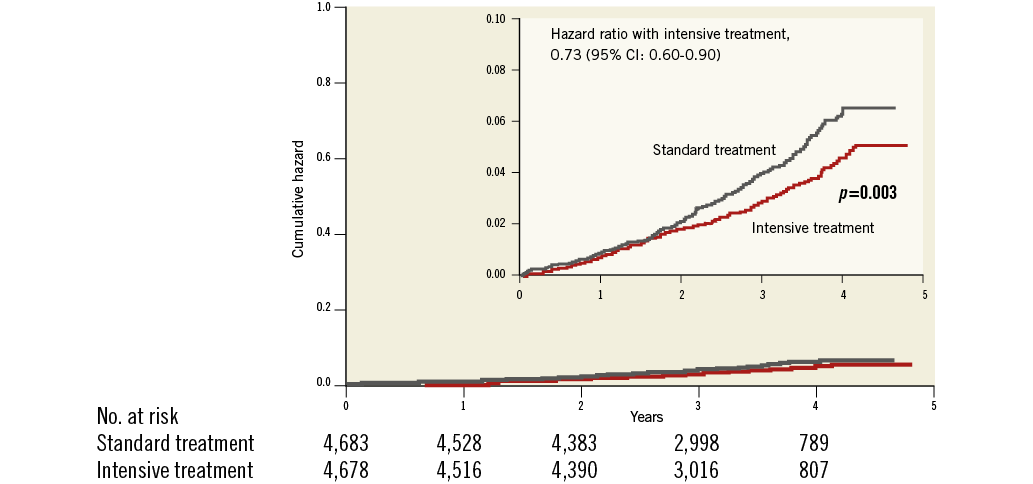
Figure 1. Death from any cause. Shown are the cumulative hazards for death from any cause (modified from SPRINT1).
Discussion and audience interaction
Although the SPRINT results support the notion “the lower the better”, consideration of the individual risks and benefits of intensive BP control need to be personalised for each individual patient based on their history and concurrent medication. Several key topics of the trial were discussed extensively during the session.
– Normotensive patients with baseline SBP <140 mmHg, but enriched by several risk factors, appear to benefit from antihypertensive therapy comparable to patients with higher baseline BP. This certainly needs further scientific evaluation as the question arises whether BP-lowering therapy should be initiated according to cardiovascular risk rather than BP levels.
– In older patients (adults aged 60 years or older) the recent US JNC-8 guidelines recommend an SBP treatment target of 150 mmHg. This recommendation seems outdated given the SPRINT findings that older as well as younger patients benefit from tight BP control. Nevertheless, especially in elderly patients, frailty and risk of injurious falls need to be taken into consideration before coming to treatment decisions. Results pertaining to cognitive outcomes and quality of life are still awaited.
– As noted above, patients with prior stroke and patients with diabetes were excluded from SPRINT. Therefore, the transferability of the findings to hypertensive patients suffering from these two frequent comorbidities is not answered by the SPRINT study.
– Increased medication and more frequent follow-up visits will be needed to achieve intensive SBP control (average number of medications intensive vs. standard treatment: 1.8 vs. 2.8). The side effects and tolerability of polypharmacy especially over time and potential for non-compliance with increasing complexity of medication regimens should be kept in mind. Careful monitoring will probably entail greater clinical scrutiny with regular assessment of renal function and electrolyte levels (participants in the intensive treatment group in the trial were seen every month until the goal was achieved).
– While drawing conclusions from the findings for everyday clinical practice, one also has to focus on the BP measurement technique that was utilised in SPRINT, as this is substantially different compared with trials of similar design and aims7. Indeed, unattended BP measurements were obtained by automated devices, without an observer being present in the room. This technique has been shown in previous studies to be comparable to or even lower than daytime ambulatory SBP, and thus up to 20 mmHg lower than the SBP values measured with the conventional auscultatory method8. In other words, this would translate to a BP goal of <140 mmHg in the intensive treatment group and ~155 mmHg in the standard treatment group, respectively, which is similar to current guideline recommendations.
The Chairperson’s conclusion: where do we stand now?
The results of SPRINT reiterate the importance of BP control as an effective approach to reduce the complications of hypertension such as heart failure, and cardiovascular death. After a lively discussion, the participants agreed that treatment decision in clinical practice should be based on an individual risk-benefit ratio and, especially in high-risk individuals, tight BP control appears to be a life-saving approach. As a final word, the following was stated: “given SPRINT results, there is no excuse for not treating elevated BP at least according to the current guideline recommendations”.
Conflict of interest statement
The authors have no conflicts of interest to declare.

