Abstract
Aims: To evaluate the clinical efficacy of the coronary sinus (CS) Reducer in attenuating angina severity in patients suffering from severe refractory angina.
Methods and results: Patients with refractory angina, objective evidence of myocardial ischaemia and no option for revascularisation were treated with CS Reducer implantation at two medical centres. Six-month follow-up evaluation consisted of clinical assessment of angina severity. Objective assessment of ischaemia at six-month follow-up was performed in one of the two centres. Successful CS Reducer implantation was achieved in 21 of 23 eligible patients, at both centres. No device-related adverse effects were observed during the procedure or the follow-up period. Canadian Cardiovascular Society (CCS) score diminished from a mean of 3.3 at baseline to 2.0 at six months (n=20, p<0.01), exercise duration was prolonged from 3:16 to 5:16 min (min:sec; n=8, p=0.05). Thallium SPECT summed stress score and summed difference score were both reduced (n=9, 21.5±10 vs.13.2±9, p=0.01, and 11.1±6 vs. 4.7±4, p=0.007, respectively). Wall motion score index at peak dobutamine infusion was also significantly improved (n=8, 1.9±0.4 vs. 1.4±0.4, p=0.046).
Conclusions: CS Reducer implantation was safe and resulted in significant improvement of angina class. The results of the ongoing randomised sham-control trial will address the concern regarding the possible placebo effect, and hopefully further support our encouraging observations.
Introduction
Refractory angina pectoris is a major clinical challenge in contemporary cardiovascular medicine1. Alongside the remarkable progress in therapeutic strategies intended for patients with coronary artery disease (CAD), a growing number of patients who suffer from severe and diffuse CAD not amenable for revascularisation continue to experience severe debilitating angina despite optimal medical therapy. These patients are often labelled as “no option” patients. Controversy remains regarding the incidence and prevalence of refractory angina. Data derived from cardiac catheterisation laboratory registries showed that 6-12% of patients referred to angiography with evidence of ischaemia were ineligible for traditional revascularisation2-5. This rate would imply 100,000 to 200,000 new patients identified per year in the USA6.
Despite the disabling symptoms, recent studies have shown that the life expectancy of patients with refractory angina is not significantly inferior to that of other patients with stable/chronic ischaemic heart disease6. It is therefore now well accepted that the goal of therapy should mostly be directed at improving these patients’ quality of life rather than extending their lifespan.
The coronary sinus (CS) Reducer™ (Neovasc Inc., Vancouver, B.C., Canada) has emerged as a therapeutic strategy for patients with refractory angina pectoris7. The Reducer is a stainless steel, balloon-expandable, hourglass-shaped stent, designed to be implanted in the CS to create a controlled narrowing of the lumen, leading to increase in coronary venous pressure (Figure 1). By elevating backward pressure and dilating subendocardial capillaries the Reducer reduces resistance to flow and restores to normal the endocardial/epicardial blood-flow ratio which is pathologically impaired in the ischaemic myocardium.
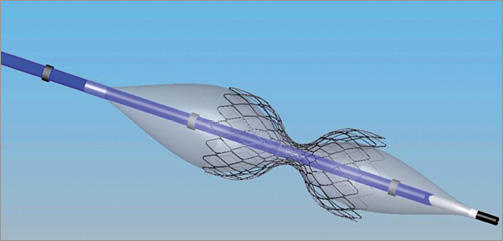
Figure 1. The coronary sinus Reducer on the dedicated balloon and delivery system.
Following preclinical experiments which demonstrated ischaemia attenuation six weeks after CS Reducer implantation in a porcine model of myocardial ischaemia (Figure 2), the first-in-man study proved the safety and feasibility of the CS Reducer7. The CS Reducer is an approved device-based therapy for refractory angina in Europe.
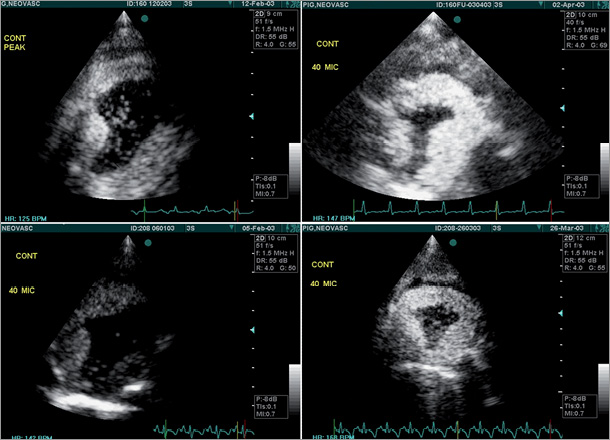
Figure 2. Contrast perfusion dobutamine echo performed in 2 pigs with reversible myocardial ischaemia of the left circumflex coronary artery territory. Left panels: baseline contrast perfusion echo at peak dobutamine infusion showing large area of ischaemia (filling defect) in the lateral wall of the left ventricle of the 2 pigs. Right panels: 6 weeks after CS Reducer implantation, contrast perfusion echo at peak dobutamine infusion and a higher heart rate demonstrate marked improvement in myocardial ischaemia in both pigs.
We describe here the clinical follow-up results from two clinical centres of CS Reducer implantation in patients treated for refractory angina. In these two medical centres the Reducer is used for the treatment of patients suffering from severe angina pectoris with objective evidence of myocardial ischaemia who are non-candidates for surgical or percutaneous coronary revascularisation.
Methods
We report here the results of the six-month clinical follow-up of the first 23 patients treated with CS Reducer implantation in two medical centres: Antwerp Cardiovascular Center ZNA Middelheim, Antwerp, Belgium, and Tel Aviv Medical Center, Tel Aviv, Israel. All participants signed a written informed consent for the treatment which has the approval of the institutional ethics committee of the Tel Aviv Medical Center, and of the Middelheim Cardiovascular Center.
PATIENT SELECTION
Patients suffering from coronary artery disease and severe angina pectoris (CCS class II-IV) despite optimal medical therapy, with objective evidence of myocardial ischaemia and ejection fraction ≥25%, who were non-candidates for surgical or percutaneous coronary revascularisation, were eligible for CS Reducer implantation.
All patients had angiographic evidence of severe obstructive coronary artery disease not suitable for revascularisation.
Excluded were patients with: 1) recent (within three months) myocardial infarction, 2) recent (within six months) PCI or CABG, 3) life-threatening rhythm disorders or any rhythm disorders that would require placement of an internal defibrillator and/or pacemaker, 4) decompensated heart failure, 5) severe valvular heart disease, 6) pacemaker or other CS electrode, 7) patients who had undergone tricuspid valve replacement or repair, and 8) patients with mean right atrial pressure higher than 15 mmHg.
THE DEVICE
The CS Reducer is a stainless steel balloon-expandable, hourglass-shaped stent, designed to be introduced into the CS through the right internal jugular vein. A few weeks following implantation the Reducer is covered by tissue, and only then does the Reducer establish a controlled narrowing of the CS. The diameter at its mid portion is 3 mm, and it can reach a diameter of 7-13 mm at both ends, using inflation pressure of 2 to 4 bars. The expansion of the Reducer is asymmetric in such a way that the proximal end expands more than the distal end, to accommodate the tapering diameter of the coronary sinus.
THE PROCEDURE
Patients were pretreated with aspirin and clopidogrel for a week prior to the procedure. Under local anaesthesia a 6 Fr diagnostic multipurpose or AL1 catheter was introduced into the CS through the right internal jugular vein under fluoroscopic guidance. Right atrial pressure was measured and was followed by angiography of the CS in 30 degrees left anterior oblique angulation. The optimal site for implantation was determined according to the vessel diameters and in order to avoid side branch bifurcation. CS diameters were determined by quantitative coronary angiography (QCA). The diagnostic catheter was then replaced (over the wire) with a preshaped 9 Fr guiding catheter, and IV heparin was administered. The Reducer, which comes preloaded on the balloon, was then introduced over the wire in the guiding catheter into the CS, positioned at the desired site, and implanted by inflating the delivery balloon to achieve slight oversizing. The desired implantation site is about 1-2 cm from the CS ostium. Post-implantation angiography was performed to ensure appropriate implantation, patency, and appropriate reduction of the lumen’s diameter (Figure 3).
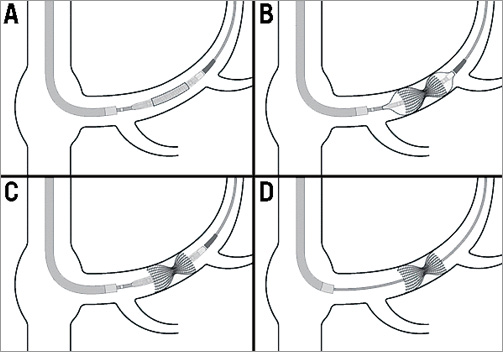
Figure 3. Schematic illustration describing the implantation process of the CS Reducer.
SCREENING AND FOLLOW-UP EVALUATION
All patients underwent a full clinical assessment, with determination of CCS class before implantation.
Objective screening evaluation consisted of one or more of the following: a treadmill stress test, a radionuclide perfusion study and/or a dobutamine echocardiography before implantation of the Reducer.
Follow-up evaluation consisted of clinical evaluation for CCS class determination at one and six months following CS Reducer implantation for all patients.
The evaluation of objective parameters at six months was performed only in one centre and the analysis of the objective measures of ischaemia was performed only for patients with available data both at baseline and at six-month follow-up.
TREADMILL EXERCISE TEST
The parameters that were inserted into the analysis were: total exercise duration, time to ST depression, METS and Duke Score.
ECHO DOBUTAMINE
A 16-segment quantitative analysis was used. The wall motion of each segment at rest and during peak dobutamine infusion was graded according to a score (1 - normal, 2 - hypokinetic, 3 - akinetic, 4 - dyskinetic, 5 - aneurysmatic)8. The summed wall motion scores of myocardial segments divided by the number of segments was defined as wall motion score index (WMSI). Baseline and six-month WMSI at rest and stress were compared.
RADIONUCLIDE PERFUSION SCAN
A 17-segment model of the left ventricle (LV) and a five-point semi-quantitative perfusion score (0 - normal to 4 - absent) were used.
The following semi-quantitative parameters of perfusion abnormalities were calculated: 1) summed rest score (SRS), calculated as the sum of the individual segment scores on the rest scan, 2) summed stress score (SSS), calculated as the sum of the individual segment scores on the stress scan, and 3) summed difference score (SDS), calculated as the difference between the SSS and the SRS and indicative of the composite of the extent and severity of ischaemia9. The SSS, SRS and SDS of all patients at baseline and six months following the procedure were compared.
STATISTICAL ANALYSIS
All data were displayed as mean (±standard deviation) for continuous variables, and as the number (percentage) of patients in each group for categorical variables. The Wilcoxon test was used to evaluate the statistical significance between baseline and six-month parameters. All of the analyses were considered significant at a two-tailed p-value of <0.05. The SPSS statistical package was used to perform all statistical evaluation (SPSS, Chicago, IL, USA).
Results
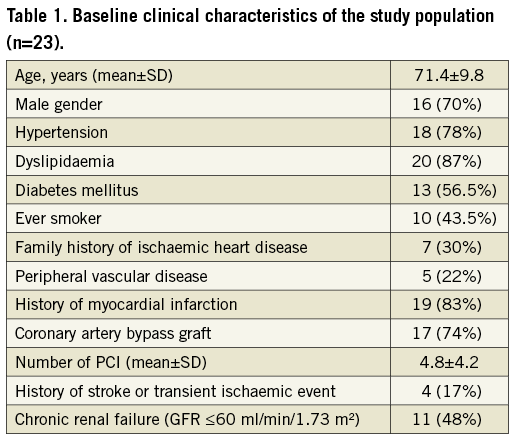
Twenty-three patients were selected for the treatment, 14 in Tel Aviv, and nine in Antwerp. Failure to implant a Reducer occurred in two patients due to unsuitable anatomy of the CS. One patient was lost to follow-up for the six-month evaluation. Therefore, the final analysis for CCS class was performed on 20 patients. Baseline clinical characteristics of the patients are shown in Table 1. Mean age was 71.4±9.8 years (range 52-88; male 70%). The study population was characterised by a high prevalence of cardiovascular risk factors including hypertension, dyslipidaemia and diabetes mellitus in 78%, 87% and 56.5% of patients, respectively. A history of myocardial infarction was present in 83% of patients, and 17 (74%) patients had a history of CABG surgery.
SUCCESS RATE AND SAFETY OF THE PROCEDURE
The Reducer implantation was successful in 21 (91%) patients. As mentioned before, the procedure failed in two patients due to an unsuitable CS anatomy. In these two patients no procedure-related complications were observed immediately after the procedure and at one-year follow-up. One of these two patients died a year after the procedure following a prolonged hospitalisation due to heart failure. Among the remaining 21 patients the procedure was uneventful and no procedure-related complications were observed during a mean follow-up period of 11.4±5 months (median 12, CI: 6-30). One patient was lost to follow-up for the six-month evaluation, but was alive and feeling well as per telephone conversation. During the follow-up period four patients underwent coronary angiography due to angina. Two of these patients were treated by PCI for new coronary lesions, one patient was referred to CABG for progression of his disease, and one patient was treated medically.
CCS CLASS
Angina score expressed by CCS class decreased significantly six months following Reducer implantation from a mean of 3.35±0.6 to 2.0±1 (n=20, p<0.001). Seventeen (85%) patients reported relief of symptoms following the procedure (Figure 4). Clinical improvement was, in most cases, reported starting a few weeks following the procedure, and maintained at one-year follow-up.
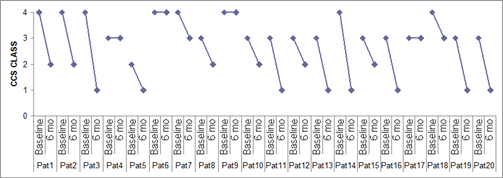
Figure 4. CCS class of all registry patients at baseline and 6 months following CS Reducer implantation.
TREADMILL STRESS TEST
The results of the exercise stress test are presented in Table 2. Exercise duration as well as time to ST depression were prolonged (3:16±1:48 vs. 5:16±1:14 min, p=0.05, and 1:47±1:12 vs. 3:57±2 min, p=0.59, respectively). METS increased and the Duke Score was also improved (4±1.8 vs. 5±1.3 and –10.8±9 vs. –6.3±7, p=0.17, respectively) but neither reached statistical significance.
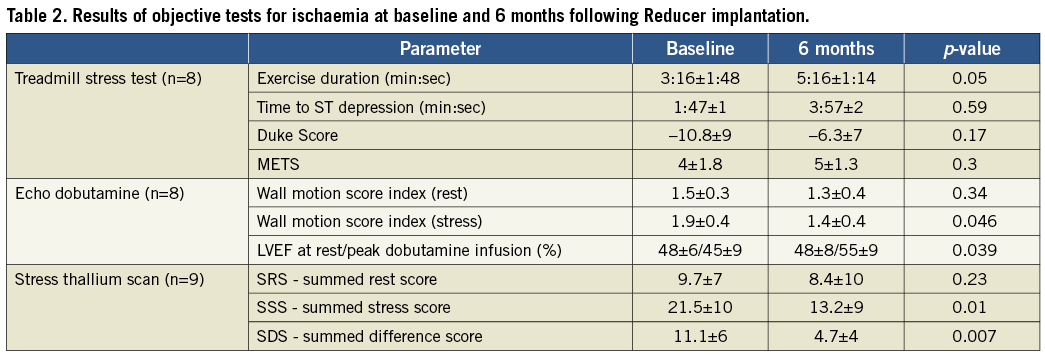
DOBUTAMINE STRESS TEST
Six months following the Reducer implantation WMSI at rest remained unchanged (1.5±0.3 vs. 1.3±0.4, p=0.4), while WMSI at stress improved significantly (1.9±0.4 vs. 1.4±0.4, p=0.046). Furthermore, while at baseline echo studies ejection fraction failed to increase and even decreased, six months following the procedure ejection fraction during dobutamine infusion increased significantly from 48.9% to 55% (p<0.04) (Table 2).
THALLIUM STRESS SCAN
The results of the thallium stress scans are presented in Table 2. As shown in the table, SRS remained unchanged six months following the procedure (9.7±7 vs. 8.4±10, p=0.23) while SSS and SDS were both significantly reduced, suggesting an improvement in the extent and severity of the ischaemic segments (21.5±10 vs.13.2±9, p=0.01, and 11.1±6 vs. 4.7±4, p=0.007, respectively).
Discussion
Coronary sinus Reducer implantation is an approved therapy for severe refractory angina in Europe. We report here the six-month follow-up clinical results of the first 23 patients treated with CS Reducer implantation. This report combines the results from two medical centres in which the CS Reducer is currently used for the treatment of such “no option” refractory angina patients.
Our results show that, in this small group of patients, implantation of the CS Reducer was associated with a significant improvement in CCS class as well as in the objective parameters of ischaemia, and that it was not associated with any adverse effects. The prevalence of refractory angina pectoris is continuing to rise alongside the ageing of the population and the increase in life expectancy of patients with ischaemic heart disease. Data on the incidence and prevalence of refractory angina are scarce and are derived mainly from cardiac catheterisation laboratory registries. According to these data, 9-14% of patients with angina and evidence of ischaemia are not candidates for revascularisation6. Patients suffering from refractory angina report a poor quality of life and a high level of depression and anxiety due to their debilitating disease. Recent reports have emphasised the need for aiming to improve the quality of life of these patients, as their mortality rates seem to be lower than previously reported and similar to other patients with stable ischaemic heart disease6. Many therapeutic strategies have been studied trying to relieve symptoms in this group of patients: spinal cord stimulation10,11, enhanced external counterpulsation12-15, myocardial laser revascularisation16-20 and others1,21-23; none of these strategies has so far become a standard of care.
The CS Reducer was designed to create a controlled narrowing of the CS, which is the final passage of the cardiac venous drainage, leading to a slight increase in coronary venous pressure. The suggested anti-ischaemic effect of the CS Reducer is based on the hypothesis described by Camici et al24, according to which, in a normal heart, during exercise there is a sympathetically mediated constriction of subepicardial vessels which leads to a preferential blood flow towards the subendocardial capillaries. In patients with obstructive coronary artery disease this physiologic compensatory mechanism is dysfunctional24. In addition, in the presence of ischaemia, impaired contractility and elevated left ventricle end-diastolic pressure (LVEDP) exert an external pressure on the subendocardial capillaries which further increases the resistance to flow to the subendocardium, leading to a vicious cycle of worsening subendocardial ischaemia. Elevated CS pressure causing backwards pressure elevation in the venules and capillaries will result in a slight dilatation of the capillaries’ diameter and a significant reduction of the resistance to flow. As a consequence of the reduction in subendocardial capillary resistance, the normal subepicardial to subendocardial blood flow ratio will be restored25. The result of this process is an enhancement of blood flow to the ischaemic subendocardial layers of the myocardium, which will improve contraction, reduce LVEDP and lead to symptoms relief.
In order to examine the validity of the presumed mechanism of action of the CS Reducer, a set of preclinical experiments and a first-in-man safety study were previously conducted with promising results7. The present report provides further evidence regarding the efficacy of the CS Reducer as a therapeutic strategy for patients suffering from refractory angina. Most of our patients reported a relief in symptoms following the procedure. The clinical improvement was felt, as expected, only a few weeks following the procedure and not immediately after implantation, as the narrowing of the CS is achieved only after complete tissue coverage and endothelialisation of the metallic struts of the device, which indeed may take several weeks. Only four patients reported no improvement following Reducer implantation. Two of these non-responders went through additional coronary angiography that demonstrated new obstructive coronary lesions, representing a progression of their basic atherosclerotic disease. These two patients were thereafter treated successfully by PCI.
Regarding the objective parameters of ischaemia evaluated in our study, although available only in a small number of patients, an improvement in the extent and severity of ischaemia in both echo dobutamine and thallium stress scan was observed. Moreover, an improvement in ejection fraction during peak dobutamine infusion was demonstrated six months following the Reducer implantation. These findings support our hypothesis that the controlled narrowing of the CS accompanied by an increase in coronary venous pressure lead to improved myocardial perfusion during stress with a consequent reduction of ischaemia.
It should be noted that the final results of the COSIRA (COronary SInus Reducer for Treatment of Refractory Angina) clinical trial26, a prospective, multicentre, randomised, double-blind, sham-controlled study are expected to be published within the next three months. The results of this study will hopefully further support our preliminary findings.
Limitations
First, the observational nature of this study precluded us from attenuating the placebo effect, which was widely reported in previous refractory angina studies. However, the objective improvement demonstrated in this study makes it reasonable to suggest that the clinical benefit attributed to the Reducer outweighs the placebo effect. Second, due to the small number of patients, some potentially important changes between baseline and follow-up evaluations did not reach statistical significance.
Conclusions
Our initial clinical experience with CS Reducer implantation as a treatment for “no option” refractory angina patients suggests a significant improvement in angina severity as well as in the objective parameters of ischaemia. The results of the randomised placebo control trial which are expected shortly will address the possible placebo effect, and will hopefully support our promising observations establishing the CS Reducer as a device-based therapy for refractory angina.
| Impact on daily practice The worldwide prevalence of patients with refractory angina is growing and new therapeutic options are needed. The coronary sinus Reducer is a device-based therapy for patients with severe angina, refractory to medical treatment. The Reducer is a potential breakthrough product. It can relieve myocardial ischaemia and improve the quality of life of patients who are disabled and symptomatic despite optimal medical therapy, and who cannot be treated with revascularisation procedures. This endoluminal, hourglass-shaped, balloon-expandable stainless steel device is percutaneously implanted in the CS to create a narrowing leading to increased CS pressures and modified myocardial blood flow. The implantation procedure is simple, safe and straightforward using a transvenous approach. |
Conflict of interest statement
Shmuel Banai is the Medical Director of Neovasc Inc. Stefan Verheye is the principal investigator for the COSIRA (COronary SInus Reducer for Treatment of Refractory Angina) clinical trial. Marc Schwartz is an employee of Neovasc Inc. The other authors have no conflicts of interest to declare.

