Abstract
Background: The REDUCER-I study is a prospective (with a retrospective component), open-label, multi-centre, international, post-market study, which collects long-term data of patients with refractory angina treated with the Reducer. Here we present the overall clinical outcomes of the first 228 patients enrolled.
Aims: The aim of this study is to examine the safety and efficacy of the coronary sinus (CS) Reducer in improving angina severity and quality of life in patients suffering from angina pectoris, refractory to medical and interventional therapies.
Methods: REDUCER-I is a multicentre, non-randomised observational study. Enrolled patients had refractory angina pectoris Canadian Cardiovascular Society (CCS) class II-IV and were treated with Reducer implantation.
Results: In the first 228 patients (81% male, 68.3±9.6 years), the procedural success rate was 99%, with only one adjudicated possible procedural or device-related MACE. Mean CCS class decreased from 2.8±0.6 at baseline, to 1.8±0.7 at two years. Improvement in ≥1 CCS class was observed in 82%, and in ≥2 CCS classes in 31% of patients at two years. At baseline, 70% of the cohort were reported to be in CCS class III-IV; this portion was reduced to 15% at follow-up. Additional measured parameters of functional class and quality of life were also improved.
Conclusions: Interim results from the ongoing REDUCER-I study confirm the high safety profile of this therapy in patients suffering from refractory angina. The results also demonstrate sustained improvement in angina severity and in quality of life up to two years.
Introduction
Chronic disabling angina, refractory to medical and interventional therapies, is a common medical condition, and a major public health problem1,2,3,4. It is common not only in patients who are not good candidates for revascularisation, but also in patients following successful revascularisation, and in patients with microvascular dysfunction.
Refractory angina correlates with increased healthcare resource utilisation, reflected by increased use of anti-anginal drugs, frequent re-hospitalisations and revascularisation attempts, and by a higher amount of sick leave among patients who are still actively working. In addition, refractory angina is associated with psychological morbidity and depression5.
The mortality rate in refractory angina patients is around 4% per year6,7. Considering the relatively favourable prognosis of these patients, it is clear that the goal of therapy should be primarily to target improving quality of life and angina symptoms, rather than prolonging life.
The coronary sinus (CS) Reducer™ (Neovasc Medical, Inc., Richmond, BC, Canada) is a device-based therapy for the treatment of refractory angina2. It is designed to improve quality of life and functional capacity by reducing angina burden8,9,10,11,12,13,14,15.
The REDUCER-I study was planned to confirm the safety and improvement in patients’ symptoms, functional status, and quality of life. It is a prospective (with a retrospective component), open-label, multicentre, international, post-market study, which collects long-term data on patients with refractory angina treated with the Reducer. This study will enrol up to 400 patients, at up to 40 centres. Here we present the overall clinical outcomes of the first 228 patients enrolled at 20 medical centres with up to two-year follow-up.
Methods
STUDY DESIGN AND POPULATION
REDUCER-I (NCT02710435) is a multicentre, international, non-randomised, open-label, two-arm observational study conducted at medical centres in Europe. Centres currently participating are listed in Supplementary Table 1. A flow chart of subjects enrolled into the study is shown in Supplementary Figure 1. Patients with chronic angina, Canadian Cardiovascular Society (CCS) class II-IV with no or limited revascularisation options undergo implantation of a Reducer in the CS, in two enrolment arms:
Arm 1. Enrols patients prospectively. All patients show objective evidence of myocardial ischaemia at baseline and are followed at 30 days, 6 and 12 months post implant, and annually up to 5 years after treatment.
Arm 2. Patients who were previously treated with the Reducer during the COSIRA study or under CE mark were invited to participate in this long-term follow-up study. Data previously collected in the COSIRA study (at baseline, procedure, 30 days and 6 months post implant) were included in this arm, as well as data that have been collected prospectively annually up to 5 years post implant.
Detailed inclusion and exclusion criteria are presented in Supplementary Table 2.
The protocol and consent form were approved by an ethics committee representing each investigational site. Signed, written, informed consent was obtained prior to enrolment.
STUDY ENDPOINTS
The primary efficacy endpoint is the percentage of patients who experience improvement in their angina symptoms defined as a reduction in CCS grade at 6 months as compared to baseline.
The primary safety endpoints are the rate of occurrence of device- and/or procedure-related periprocedural serious adverse events (SAEs), and major adverse cardiac events (MACE), a composite of cardiac death, major stroke, and myocardial infarction (MI) up to 30 days post implant.
The secondary safety and efficacy endpoint is the percentage of patients who experience a reduction in CCS grade and the rate of MACE at 12 months and annually up to 5 years post implant as compared to baseline.
Other observational methods included outcomes of exercise tolerance test (ETT), six-minute walk test (6MWT), change in Seattle Angina Questionnaire (SAQ)16 and EQ-5D-5L17, use of anti-anginal medications, and number of emergency department (ED) visits, as described in detail in Supplementary Appendix 1.
CLINICAL EVALUATIONS
Arm 1. Baseline evaluations performed within 30 days prior to treatment included the following: medical history, physical examination, listing of documented ED visits due to angina within the 12-month period prior to enrolment, full listing of current cardiac medications and dosages, CCS angina assessment, SAQ, EQ-5D-5L, ETT, 6MWT, and resting electrocardiogram (ECG) within 24 hours prior to procedure.
The 30-day (±7 days) follow-up visit was conducted by phone to review all protocol-defined adverse events (AEs). The 6- and 12-month follow-up evaluations consisted of a physical exam, full listing of current cardiac medications and dosages, CCS angina assessment, SAQ, EQ-5D-5L, ETT, 6MWT, a review of protocol-defined AEs, and review of all documented ED visits in the past 12 months due to angina episodes (collected at the 12-month visit only). Annual follow-ups included evaluation of CCS class, physical exam, EQ-5D-5L, SAQ and MACE.
Arm 2. Data available from the treatment arm of the COSIRA study (at baseline, procedure, 30 days and 6 months post implant) or data previously collected for patients treated with the Reducer under CE mark, including baseline evaluation, procedural data and clinical follow-ups, were included in this study. Prospective annual follow-ups were similar to Arm 1.
DEVICE AND PROCEDURE
The device and the procedure have been described in detail elsewhere9,12,18,19, and are presented in brief in Supplementary Appendix 2 and Supplementary Appendix 3.
DATA COLLECTION AND MANAGEMENT, CLINICAL EVENTS COMMITTEE
Data were collected using standardised case report forms (CRFs). Ongoing periodic monitoring visits and data review ensure the quality of clinical data across all subjects and sites. A clinical events committee (CEC) is responsible for adjudicating endpoint-related events reported during the study. CEC members are independent of both the sponsor and the study investigators and meet regularly according to a pre-determined schedule.
STATISTICAL ANALYSIS
Analyses were conducted separately for the Arm 1 (prospective) cohort and the overall cohort. Demographics and outcomes were summarised by means and standard deviations for continuous variables, and by counts and percentages for categorical variables.
To analyse changes from baseline, Bowker’s tests of symmetry were used for CCS angina class. Paired t-tests were used to analyse change from baseline in 6MWT distance, ETT parameters including exercise duration and time-to-1 mm ST-segment depression, and SAQ domains.
EQ-5D-5L and the visual analogue scale (EQ-VAS) were only collected from patients in Arm 1. Severity levels for each dimension were dichotomised as having no problems (“No problems”) or slight to extreme problems (“Problems”). To analyse change from baseline, McNemar’s tests were used for the EQ-5D-5L dimensions and paired t-tests for EQ-VAS.
Outcomes were plotted over time as percentages or means with 95% confidence intervals. All tests were two-sided, and all statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
STUDY POPULATION, PROCEDURAL SUCCESS AND SAFETY ENDPOINTS
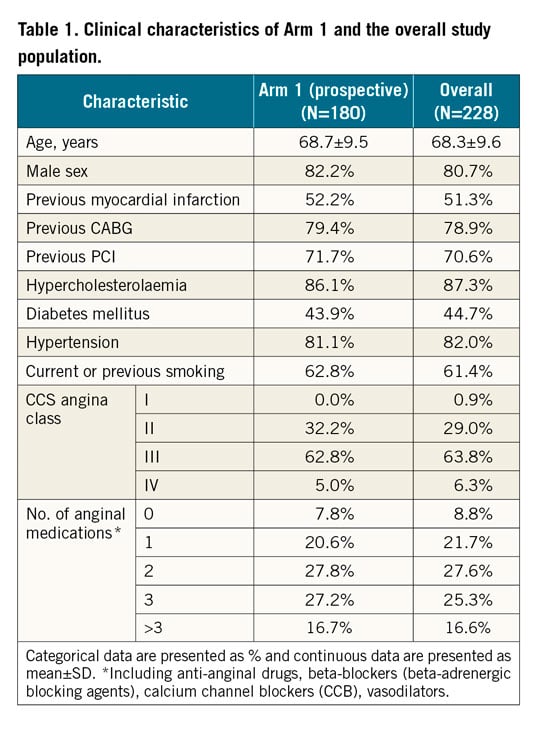
From March 2016 up to March 2020, a total of 228 patients (68.3±9.6 years, 81% male) were enrolled, with 180 in the prospective Arm 1, and 48 in Arm 2. Median follow-up time from the date of procedure for Arm 1 was 666 days (range 0.0-1,386.0). Overall, 158 patients have reached the 1-year and 111 the 2-year follow-up visit (a detailed description of completion of follow-up visits is presented in Supplementary Table 3). The study population was characterised by high rates of cardiovascular risk factors and coexisting comorbidities, as presented in Table 1.
The device was successfully implanted in 226 of the 228 patients (99%). In two patients, Reducer implantation was not successful for the following reasons: guiding catheter-induced CS dissection with no clinical sequelae in one case, and inability to find the CS ostium in the other case. In three other patients the first implantation attempt failed due either to CS dissection without clinical sequelae (one case), or to technical difficulty to engage the guiding catheter into the CS (two cases). However, in all three patients a successful implantation was accomplished in a second attempt.
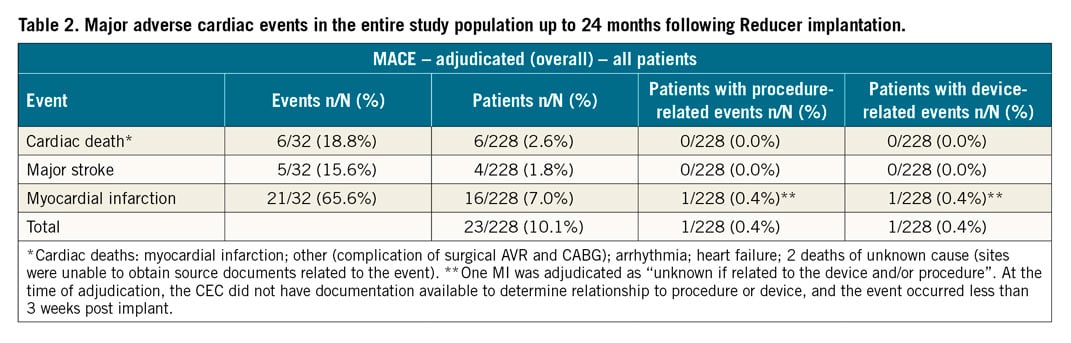
MACE at long-term follow-up are presented in Table 2. Total mortality was 5.7% (13 patients), cardiovascular death occurred in 6 patients (2.6%), and 16 patients experienced MI (7.0%). There was one periprocedural MACE recorded (an MI event) occurring less than 3 weeks post implant, adjudicated as unknown if device- or procedure-related.
EFFICACY ENDPOINT
CHANGE IN CCS ANGINA CLASS
Baseline and follow-up information regarding CCS angina class was available for 220 patients and is presented in Table 3. An improvement of ≥1 CCS class was recorded in 74% of patients (75% in Arm 1) at 1 year, and in 82% of patients (84% in Arm 1) at 2 years. An improvement of ≥2 CCS classes was reported in 31% of patients (26% in Arm 1) at 2 years after Reducer implantation (Figure 1). The rate of non-responders (patients reporting no change in CCS class) was 24% at 1 year and 17% at 2 years. Among these, 2 patients reported a worsening in CCS class at 1 year.
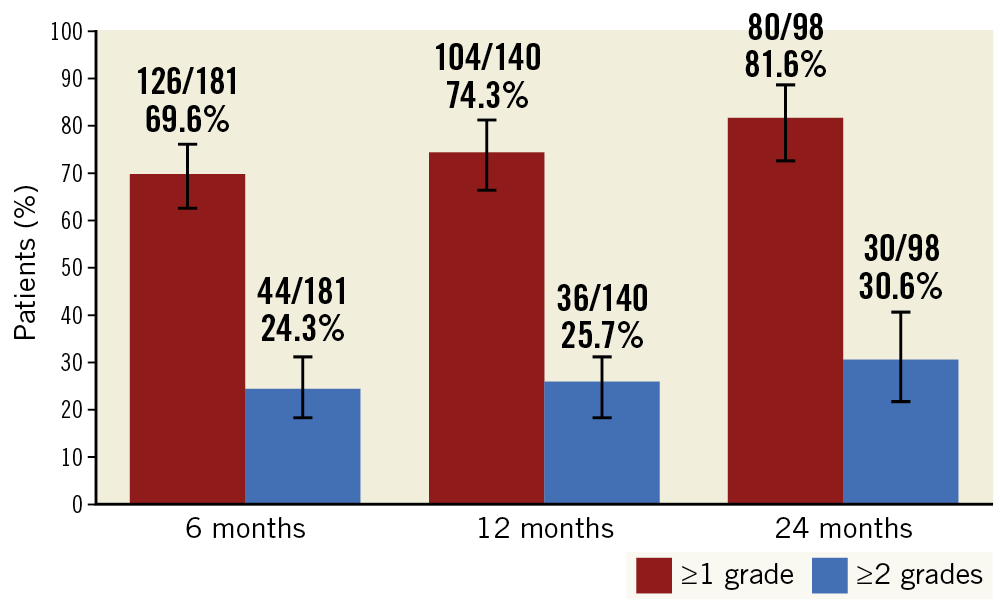
Figure 1. Improvement in CCS class at 6, 12 and 24 months following Reducer implantation.
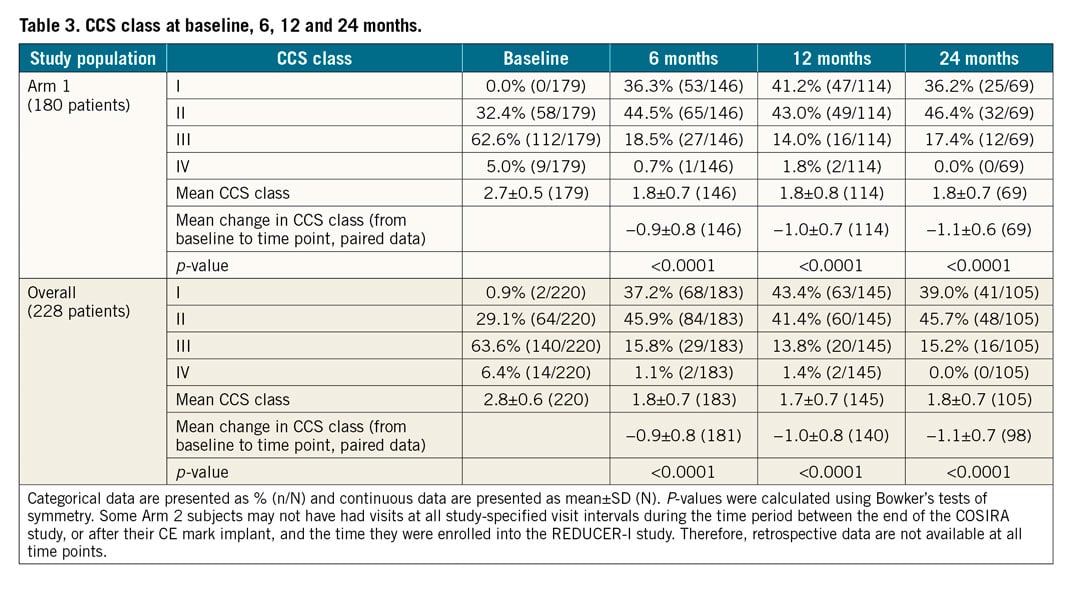
Mean CCS class improved from 2.8±0.6 at baseline to 1.8±0.7 at 6 months, 1.7±0.7 at 1 year, and 1.8±0.7 at 2 years for the whole cohort and, similarly, from 2.7±0.5 to 1.8±0.7 at 6 months, 1.8±0.8 at 1 year and 1.8±0.7 at 2 years for Arm 1 (p<0.0001, for all) (Table 3, Figure 2A, Figure 2B).
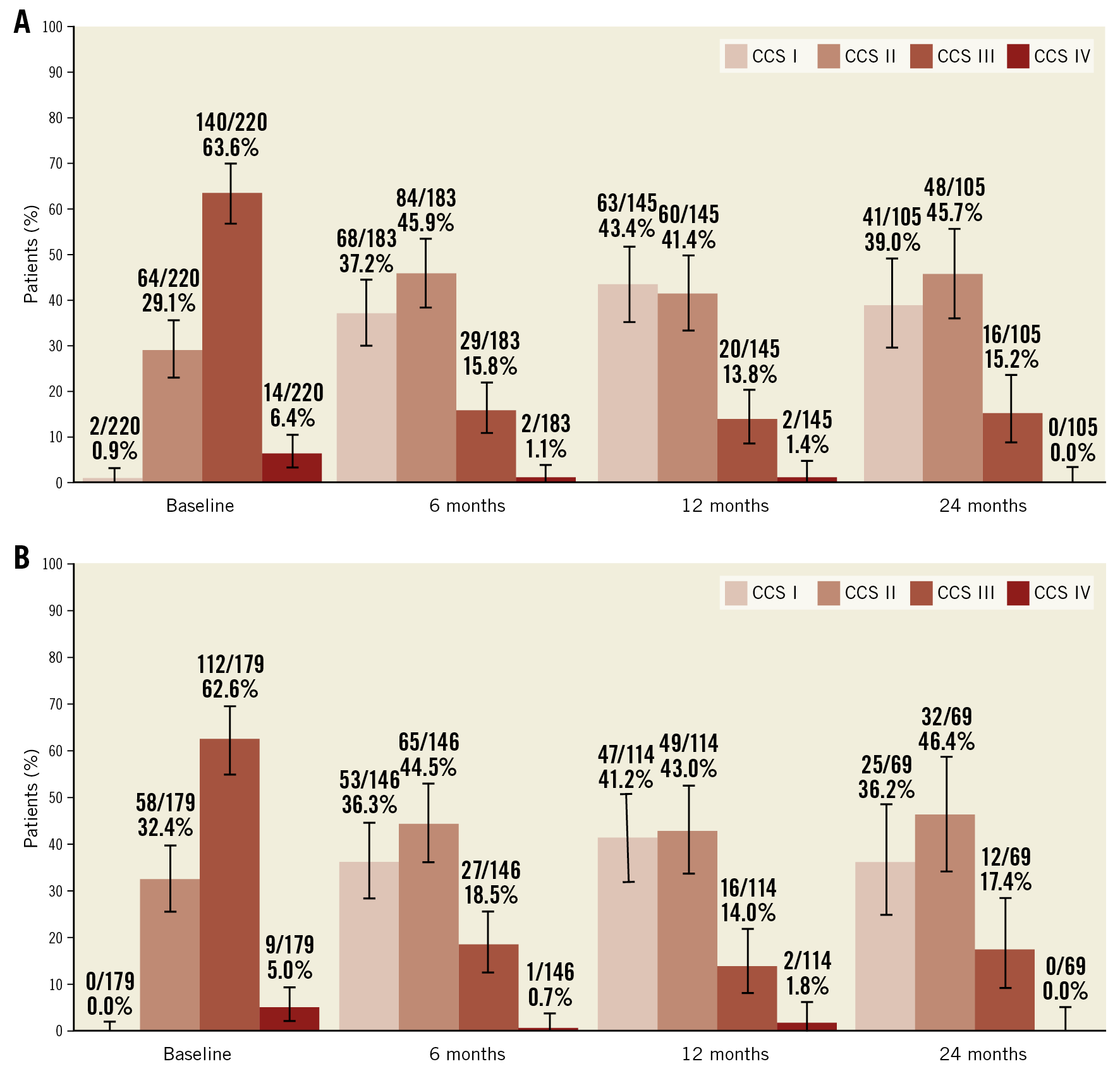
Figure 2. CCS class over time for the overall study population (A) and for Arm 1 (B).
At baseline, 70% of patients suffered from severe disabling angina (CCS class III-IV). The rate of patients with disabling angina was reduced to 15% at 1 and 2 years after treatment. This represents a 79% reduction at 1 and 2 years in the severity of angina in the subgroup of patients with the most severe angina (Figure 3). There was no change in the use of anti-anginal medications over time (3.1±1.3 at baseline and 3.0±1.3 and 3.0±1.5 at 1 (n=117) and 2 years (n=71), respectively, p>0.1 for both) (Supplementary Table 4).
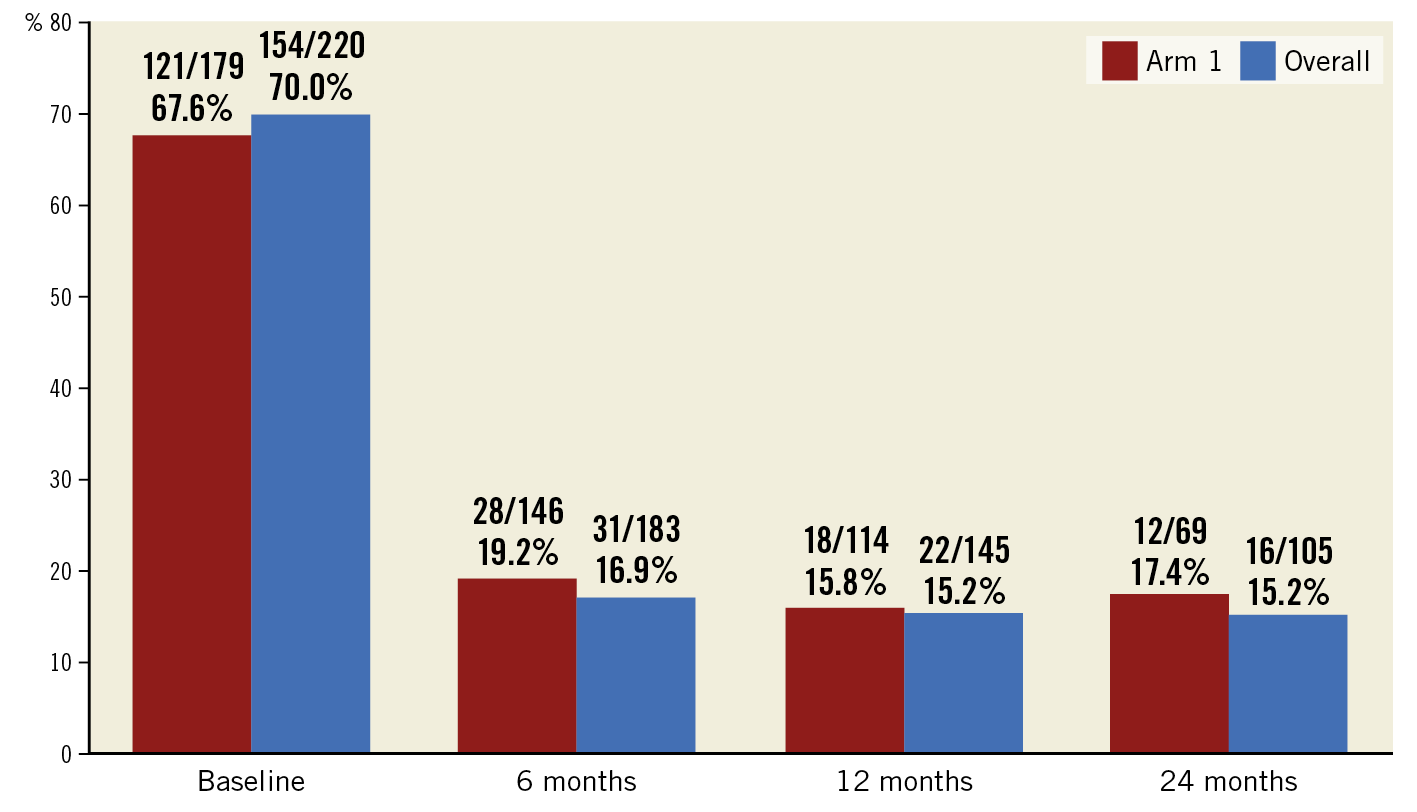
Figure 3. Percentage of patients with severe angina (CCS class III-IV) over time.
SIX-MINUTE WALK TEST AND EXERCISE TOLERANCE TEST
The results of the 6MWT and ETT are presented in Supplementary Table 5. For the entire cohort, 6MWT improved following Reducer implantation (from 327.5±118.4 m at baseline to 378.5±100.4 m and 364.3±110.9 m at 6 and 12 months, respectively; p<0.0001 for 6 months and 0.0004 for 12 months). Exercise duration increased from 359.9±165.1 seconds at baseline to 383.1±157.5 seconds at 6 months and to 409.4±165.0 seconds at 12 months (p=0.025 and p=0.0011, respectively). There was no change in time to ST segment depression at ETT.
SEATTLE ANGINA QUESTIONNAIRE, EQ-5D-5L SCORE
The results of quality-of-life assessment by the EQ-5D-5L score are available only for patients enrolled in Arm 1 and are reported in Supplementary Table 6. The percentage of patients who reported having problems in mobility and usual activities decreased at 6 and 12 months following treatment, as well as the percentage of patients reporting having anxiety and pain. EQ-VAS improved from 57.5±19.5 at baseline to 67.7±17.4 at 6 months and to 67.5±17.3 at 12 months, p<0.0001 for both.
SAQ results are presented in Supplementary Table 7. In the prospective arm, as well as in the overall study cohort, there was an improvement in all 5 domains of the questionnaire at 6 and 12 months following treatment (p<0.0001 for all). Improvement in 4 of 5 domains was sustained at 24 months.
NUMBER OF DOCUMENTED ED VISITS DUE TO ANGINA
A total of 113 patients, all enrolled in Arm 1, had documented information regarding the number of ED visits due to angina during the year prior to Reducer implantation, and during the year following treatment. Forty-seven patients (41.6%) had at least one ED visit with a total of 78 visits during the year preceding Reducer implantation, resulting in an average of 0.69±1.06 ED visits per patient (range 0-5 visits). Following treatment, only 15 patients (13.3%) had at least one ED visit with a total of 22 ED visits. The average number of visits per patient decreased in the year following Reducer implantation to 0.19±0.60 (range: 0-4 visits) (p<0.0001).
Discussion
This is the largest cohort of patients with refractory angina treated with CS narrowing. The ongoing single-arm open-label REDUCER-I study has been collecting long-term data of patients with angina undergoing Reducer implantation. The overall clinical outcomes of the first 228 patients enrolled are presented here, including the results of the prospective arm (n=180).
The main findings are the following. 1) Procedural success was very high (99%) with only one case of periprocedural MI possibly related to the device/procedure, confirming the feasibility and safety of this therapy. 2) The majority of patients experienced improvement in angina symptoms. 3) The proportion of patients with angina in minimal effort and at rest (CCS class III-IV) decreased from 70% at baseline to 15% at 1 and 2 years following treatment. 4) Angina symptoms and quality of life, as evaluated by SAQ and EQ-5D-DL scores, improved. 5) Functional capacity, as evaluated by 6MWT and exercise duration in ETT, improved. 6) The number of documented ED visits due to angina episodes decreased following Reducer implantation, while there was no change in the number of prescribed anti-anginal medications.
A large number of patients with stable coronary artery disease (CAD) continue to experience severe angina despite medical and interventional therapies1,2,3,4. Angina persists after successful revascularisation procedures in as many as 20-40% of patients during short- to medium-term follow-up, and in up to 45% after 3 years20. Because many patients with refractory angina are disabled in their daily activities, with poor quality of life, and no further treatment options, we decided to report interim results revealing the safety of a new available therapy.
CS narrowing using the Reducer has emerged as an effective therapy for patients suffering from disabling angina. In the COSIRA double-blind, sham-controlled, multicentre clinical trial, Reducer implantation was associated with significantly greater angina relief and improved quality of life compared with a sham procedure, despite a high rate of placebo effect15. The safety and efficacy of this treatment have been demonstrated in numerous clinical observational studies9,10,11,12,13,21,22,23,24,25,26.
The results of the first 228 patients of the REDUCER-I study presented here show a high procedural success rate with a high safety profile, reflecting the relative simplicity of the procedure. The net clinical benefit in the majority of treated patients is represented by improvement in CCS class, 6MWT, exercise duration, all domains of SAQ and EQ-5D-DL scores, and the number of ED visits due to angina.
Importantly, the results presented here for the prospective arm are not different from those of the retrospective arm of the study and are similar to the results of the randomised, sham-controlled COSIRA trial and other smaller, non-randomised and observational studies, further supporting the validity of the Reducer as a safe and effective therapy for patients who, until recently, were labelled as “no option” patients. Unlike the COSIRA randomised trial that enrolled only patients in CCS class III-IV, in the REDUCER-I study, patients in CCS class II were also eligible, since we encounter patients with stable angina who do not wish to be at all limited in their daily physical activities and therefore seek a medical solution.
We report a decrease in the number of ED visits following the procedure but no reduction in the prescription of anti-anginal medications. As medical treatment during follow-up was left to the discretion of the treating physicians, we can speculate that many physicians were reluctant to change existing medical therapy, even when patients became less symptomatic.
The presumed anti-ischaemic mechanism of CS pressure elevation has been described previously12,27. It is described briefly in Supplementary Appendix 4.
Limitations
First, we report the results of only the first 228 patients enrolled in a study that is continuing to enrol up to 400 patients. Second, patients included in Arm 2 were enrolled retrospectively and therefore certain bias cannot be excluded. However, we show that the outcomes in the overall cohort are similar to those of Arm 1. Third, this is an open-label unblinded study with no control group, and technicians performing the tests were not blinded to treatment. Therefore, a certain bias due to the nature of this design cannot be excluded. Furthermore, it is possible that our results were augmented by a placebo effect that has been previously recognised following treatment in patients suffering from refractory angina. Fourth, the first clinical evaluation was performed 6 months following the procedure, and therefore we do not have data regarding efficacy outcomes at 1 and 3 months. In addition, as the study is designed to enrol patients in many different European countries, with different medical-economic systems and costs, we were unable to estimate the possible reduction of medical costs following the procedure. However, we do report outcomes that affect costs (medical treatment and ED visits).
Conclusions
Based on the current interim results of this largest-to-date, open-label clinical study, Reducer implantation in patients suffering from stable refractory angina is feasible and safe. Improvement in symptoms, quality of life and functional capacity was also observed. Given the safety of the device, large, sham-controlled, confirmatory studies with objective endpoints are required to establish the efficacy of this therapy further.
|
Impact on daily practice Refractory angina is a disabling condition which affects patients’ quality of life and has a significant impact on healthcare resources. Patients suffering from refractory angina are often labelled as “no option” patients. The REDUCER-I trial is the largest-to-date prospective cohort of patients undergoing Reducer implantation for the treatment of refractory angina. Interim results of this study confirm the good safety profile of this therapy along with solid evidence of improvement in angina symptoms, physical performance, and quality of life. |
Funding
The REDUCER-I study is funded by Neovasc Inc.
Conflict of interest statement
S. Verheye, P. Agostoni and F. Giannini serve as proctors for Neovasc Inc. S. Banai is the Medical Director of Neovasc Inc. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

