Abstract
The coronary sinus Reducer (CSR) is an hourglass-shaped device which creates an artificial stenosis in the coronary sinus. Whilst placebo-controlled data show an improvement in angina, these results are unreplicated and are the subject of further confirmatory research. The mechanism of action of this unintuitive therapy is unknown. The Coronary Sinus Reducer Objective Impact on Symptoms, MRI Ischaemia, and Microvascular Resistance (ORBITA-COSMIC) trial is a randomised, placebo-controlled, double-blind trial investigating the efficacy of the CSR. Patients with (i) established epicardial coronary artery disease, (ii) angina on maximally tolerated antianginal medication, (iii) evidence of myocardial ischaemia and (iv) no further options for percutaneous coronary intervention or coronary artery bypass grafting will be enrolled. Upon enrolment, angina and quality-of-life questionnaires, treadmill exercise testing and quantitative stress perfusion cardiac magnetic resonance (CMR) imaging will be performed. Participants will record their symptoms daily on a smartphone application throughout the trial. After a 2-week symptom assessment phase, participants will be randomised in the cardiac catheterisation laboratory to CSR or a placebo procedure. After 6 months of blinded follow-up, all prerandomisation tests will be repeated. A prespecified subgroup will undergo invasive coronary physiology assessment at prerandomisation and follow-up. The primary outcome is stress myocardial blood flow on CMR. Secondary outcomes include angina frequency, quality of life and treadmill exercise time. (ClinicalTrials.gov: NCT04892537)
The coronary sinus Reducer (CSR; Shockwave Medical [previously Neovasc]) is an hourglass-shaped, stainless-steel mesh, currently used in patients with angina, which is refractory to antianginal medication and conventional antianginal procedures including coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). It is the only currently utilised intervention for stable angina which is thought to improve myocardial ischaemia by acting on the cardiac venous circulation and has placebo-controlled evidence of angina relief12.
The Coronary Sinus Reducer for Treatment of Refractory Angina (COSIRA) study reported a placebo-controlled reduction in physician-assessed Canadian Cardiovascular Society (CCS) angina class1. However, the mechanism of action of this device is unintuitive and remains unknown. All previous antianginal therapies are considered to work by improving myocardial ischaemia. Many mechanistic theories have been offered for the CSR34. However, these have principally been derived from animal models of myocardial infarction.
In humans, the effect of the CSR on myocardial ischaemia has been investigated in a single-arm unblinded registry. While global myocardial perfusion did not change, segments that were determined to be ischaemic at baseline appeared to improve5. The impact that randomisation, a control group, placebo subtraction, blinded reporting, and quantification of myocardial blood flow (MBF) might have had on this result is unknown.
The Coronary Sinus Reducer Objective Impact on Symptoms, MRI Ischaemia and Microvascular Resistance (ORBITA-COSMIC) trial will investigate the placebo-controlled efficacy of the CSR on quantified myocardial perfusion and stable angina.
Methods
STUDY DESIGN
ORBITA-COSMIC is a randomised, placebo-controlled trial of the CSR investigating the efficacy of the CSR on myocardial perfusion and angina. It is registered on ClinicalTrials.gov (NCT04892537) and approved by the London Research Ethics Committee (reference 21/LO/0203). The study design is shown in Figure 1. A list of the ORBITA-COSMIC investigators is included in Supplementary Appendix 1.
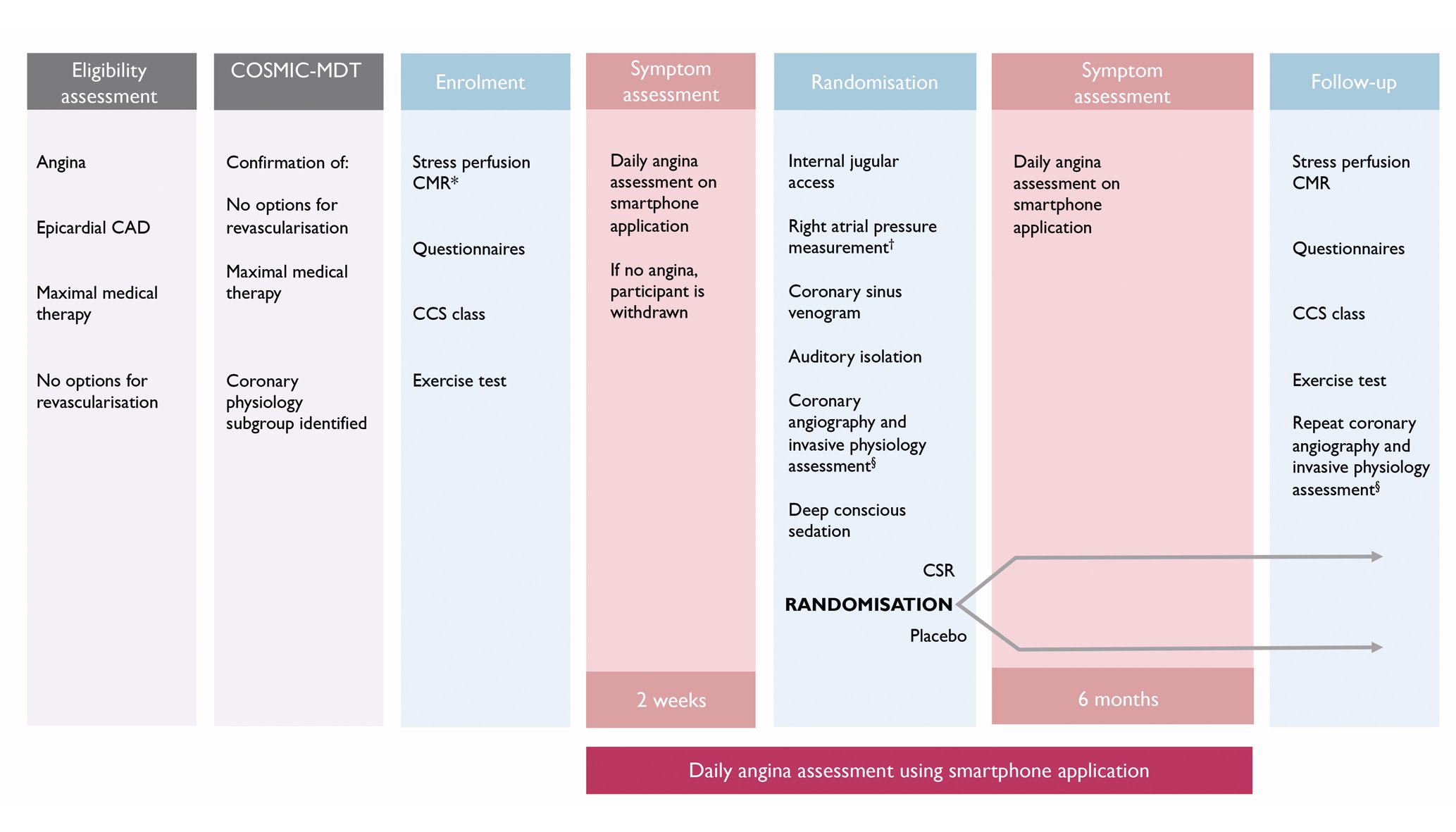
Figure 1. ORBITA-COSMIC study design. Questionnaires and CCS class assessment additionally administered immediately prior to randomisation. *If there is no ischaemia outside of the inferior wall on this scan, the participant is removed from the study at this stage and does not proceed to randomisation. †Participants with a right atrial pressure ≥15 mmHg will not proceed to randomisation and will be removed from the study. §For those patients identified as eligible during the COSMIC-MDT assessment. CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CMR: cardiac magnetic resonance; COSMIC-MDT: ORBITA-COSMIC multidisciplinary team; CSR: coronary sinus Reducer
ELIGIBILITY CRITERIA
ORBITA-COSMIC will randomise participants who have the following:
1. Angina on maximally tolerated antianginal medication,
2. Epicardial coronary artery disease (CAD),
3. Evidence of ischaemia on cardiac magnetic resonance (CMR) imaging,
4. No further options for CABG or PCI.
Angina is diagnosed by the referring clinician. Medical therapy is considered maximal when the patient is established on ≥3 antianginal medications at target doses or has documented intolerance to additional antianginals. Epicardial disease is defined as visual angiographic stenosis ≥50% in at least one major epicardial coronary artery or previous CABG or PCI. Documented ischaemia on stress perfusion CMR is required for enrolment. The absence of further options for CABG or PCI is determined by the clinical site’s Heart Team, prior to referral. These teams consist of invasive cardiologists, general cardiologists, imaging cardiologists and cardiac surgeons.
Confirmation of eligibility will be performed by the ORBITA-COSMIC multidisciplinary team (COSMIC-MDT). This team is comprised of interventional cardiologists from the trial sites, with extensive experience in managing complex CAD, including chronic total occlusions and post-CABG CAD. To progress to enrolment, the COSMIC-MDT must reach a consensus that there are no reasonable PCI or additional antianginal medication options.
The inclusion and exclusion criteria are listed in Table 1.
Table 1. ORBITA-COSMIC inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Established epicardial CAD • Angina on maximally tolerated antianginal therapy • Ischaemia on quantitative stress perfusion CMR, beyond the inferior wall • No further options for PCI or CABG |
• Age <18 years |
| • Pregnancy | |
| • Inability to consent | |
| • Recent acute coronary syndrome (≤3 months) | |
| • Recent revascularisation (≤6 weeks) | |
| • Permanent pacemaker or defibrillator leads in the right heart | |
| • Severe left ventricular impairment (LVEF <25%) | |
| • Indication for CRT | |
| • Right atrial pressure ≥15 mmHg | |
| • Life expectancy <1 year | |
| • Severe renal impairment (eGFR <15 ml/min) | |
| • Contraindication to CMR | |
| • Contraindication to adenosine | |
| • Ischaemia isolated to inferior wall | |
| • Ongoing participation in a separate interventional study | |
| CABG: coronary artery bypass grafting; CAD: coronary artery disease; CMR: cardiac magnetic resonance; CRT: cardiac resynchronisation therapy; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention | |
PRIMARY AND SECONDARY OUTCOMES
The primary outcome is the between-group difference in myocardial blood flow (MBF) on adenosine stress CMR at 6-month follow-up in the segments designated to be ischaemic at baseline. This imaging outcome will be analysed using Bayesian methodology, in which stress MBF at 6 months is conditioned on the enrolment value and treatment arm. The MBF at rest and stress will be quantified using validated perfusion mapping with automated analysis using the Gadgetron (open source framework)67891011. Enrolment and follow-up CMR will have matched adenosine dose and duration and will be performed by a blinded research team. An example quantitative CMR is shown in Figure 2. A single-arm study has suggested an improvement in the endocardial-epicardial perfusion gradient with the CSR12. This will be investigated as a secondary imaging outcome. The primary symptom outcome is the daily angina episodes component of the angina symptom score13. Outcomes are listed in Table 2.
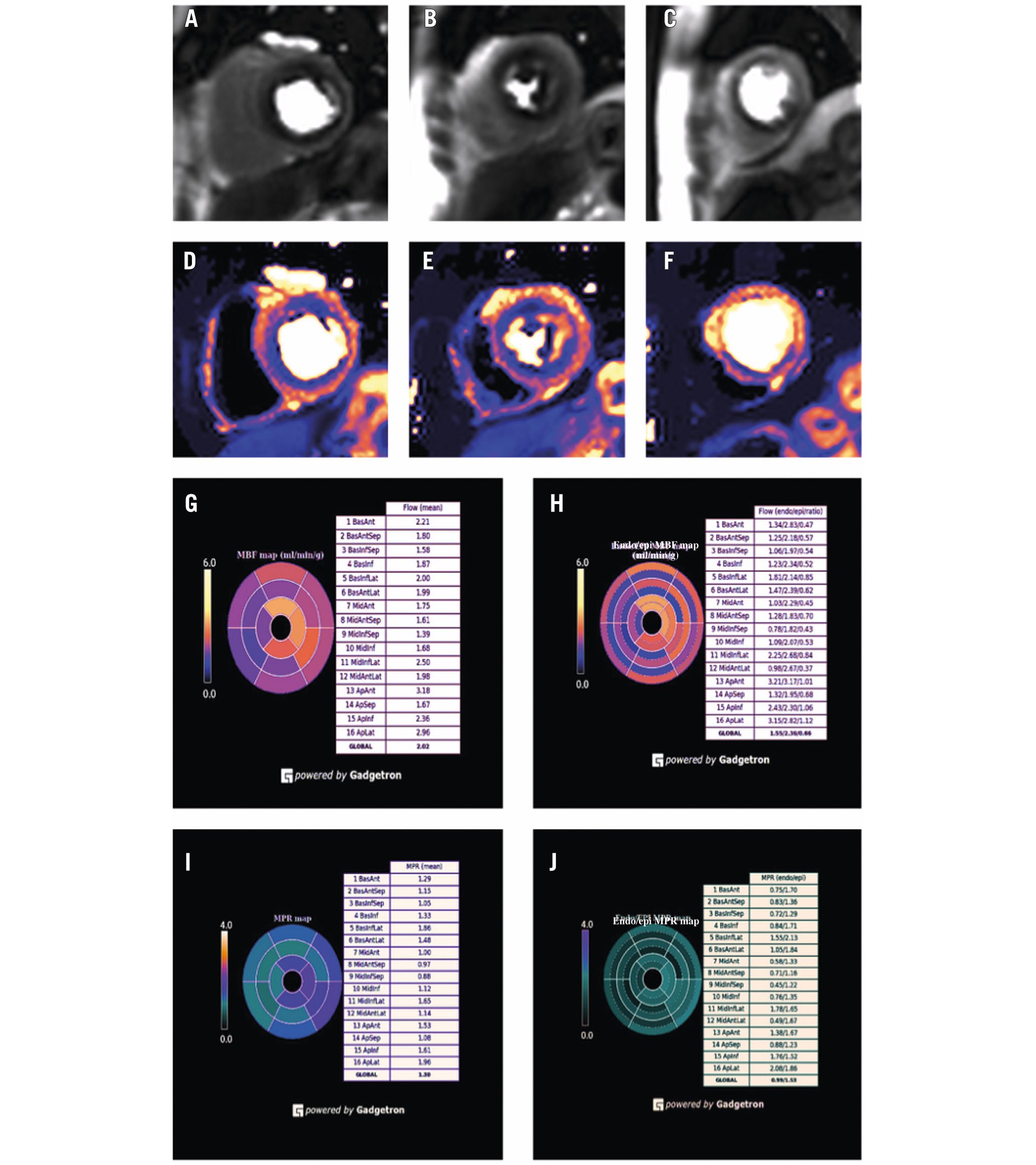
Figure 2. Quantitative perfusion mapping in a patient with refractory angina. A-C) Shows first-pass perfusion images during maximal hyperaemia induced by adenosine stress in the basal, mid-, and apical short-axis slices. There are subendocardial perfusion defects in the LAD and RCA territories. D-F) Shows perfusion maps of myocardial blood flow on a pixel-by-pixel basis in the same basal, mid-, and apical short-axis slices. G) Shows myocardial blood flow on stress by segment, and for the epicardial (epi) and endocardial (endo) component of each segment (H), provided inline during the scan in an automated bias-resistant manner using the Gadgetron framework. Myocardial perfusion reserve by segment (I) and for epicardial and endocardial components of each segment (J) are also automated and are provided after the rest perfusion images. In this case, the bull's-eye plots demonstrate reduced myocardial blood flow on stress and reduced MPR in segments in the anterior, anteroseptal, inferoseptal and inferior walls. LAD: left anterior descending artery; MPR: myocardial perfusion reserve; RCA: right coronary artery
Table 2. ORBITA-COSMIC trial outcomes.
| Study primary outcome | |
|---|---|
| CMR | MBF on CMR* |
| Imaging secondary outcomes | |
| CMR | MPR in ischaemic segments, non-ischaemic segments, and global MPR |
| Rest MBF in ischaemic segments, non-ischaemic segments, and global rest MBF | |
| Stress MBF in ischaemic segments with inferior and inferoseptal segments excluded | |
| MPR in ischaemic segments with inferior and inferoseptal segments excluded | |
| Rest MBF in ischaemic segments with inferior and inferoseptal segments excluded | |
| Endocardial-epicardial ratio of stress MBF | |
| Endocardial-epicardial ratio of MPR | |
| Endocardial-epicardial ratio of rest MBF | |
| Myocardial strain | |
| Myocardial scar burden | |
| Symptom primary outcome | |
| Patient reported | Episodes of angina component of angina symptom score |
| Symptom secondary outcomes | |
| Physician assessed | CCS |
| Patient reported | Angina symptom score |
| SAQ angina frequency | |
| SAQ angina physical limitation | |
| SAQ quality of life | |
| SAQ treatment satisfaction | |
| SAQ angina stability | |
| EQ-5D-5L descriptive system | |
| EQ-5D-5L visual analogue scale | |
| Angina-related quality of life rated by the MacNew questionnaire | |
| Other | Treadmill exercise time |
| Invasive physiology substudy outcomes | |
| Invasive physiology | Absolute flow assessed by a pressure and temperature sensor wire (PressureWire X; Abbott) and an intracoronary saline infusion catheter (RayFlow; Hexacath) |
| Absolute resistance assessed by a pressure and temperature sensor wire and an intracoronary saline infusion catheter | |
| MRR assessed by a pressure and temperature sensor wire and an intracoronary saline infusion catheter | |
| CFR assessed by a pressure and temperature sensor wire | |
| IMR assessed by a pressure and temperature sensor wire | |
| CFR assessed by a pressure and Doppler sensor wire (ComboWire; Philips) | |
| *MBF is measured during adenosine stress and will be calculated in segments which were designated ischaemic on the baseline CMR. CCS: Canadian Cardiovascular Society class; CFR: coronary flow reserve; CMR: cardiac magnetic resonance; EQ-5D-5L: EuroQol questionnaire; IMR: index of microcirculatory resistance; MBF: myocardial blood flow; MPR: myocardial perfusion reserve; MRR: microvascular resistance reserve; SAQ: Seattle Angina Questionnaire | |
ENROLMENT
At enrolment, eligibility will be rechecked, and written consent will be obtained. Symptoms will be assessed by Canadian Cardiovascular Society (CCS) class and symptom questionnaires including the Seattle Angina Questionnaire (SAQ), Rose Angina Questionnaire, EuroQol (EQ-5D-5L) and the MacNew Heart Disease Health-Related Quality of Life Questionnaire. The participants will be taught to use the ORBITA-COSMIC smartphone application for documentation of daily angina symptoms (Supplementary Appendix 2, Supplementary Table 1, Supplementary Table 2)1314. Participants will undergo treadmill exercise testing, utilising the Modified Bruce Protocol (Supplementary Appendix 3). The management of medications is described in Supplementary Appendix 4.
STRESS CMR CONFIRMATION OF ISCHAEMIA
At enrolment, all participants will undergo a quantitative perfusion CMR7 (Supplementary Appendix 5). For inclusion, ischaemia is defined as present or absent by the reporting clinical cardiologist. No minimum ischaemia threshold or a relationship between anatomical stenosis and myocardial ischaemia is required. This is to ensure that patients with a broad range of ischaemia are randomised. Patients with ischaemia involving the inferior wall will be included if they have additional ischaemia in other territories.
Scans will be assessed by a core imaging laboratory of 3 CMR experts. They will double report every CMR, blinded to all clinical data, the randomisation arm, and time point. They will determine the location of ischaemia and scar, by segment, for use in the assessment of the primary outcome.
SYMPTOM EVALUATION
Once enrolled, participants will enter the 2-week prerandomisation symptom assessment phase. If asymptomatic, they will be withdrawn. Angina will be assessed daily throughout the trial using the symptom smartphone application (Supplementary Appendix 3). Participants will have additional symptom and quality-of-life assessment performed at enrolment, prerandomisation, and follow-up.
CORONARY PHYSIOLOGY SUBGROUP
A subgroup of participants will be eligible for invasive physiological assessment if they have a patent native coronary artery which can be safely investigated. Eligibility will be determined by the COSMIC-MDT.
This subgroup will undergo invasive assessment at prerandomisation and follow-up. The aim is to measure macro- and microvascular coronary function using multiple modalities. Measurements will be preferentially performed in the left main coronary artery and the ostial right coronary artery, where coronary anatomy permits. This will be performed before sedation and randomisation and will include absolute coronary flow and resistance using continuous thermodilution with the RayFlow catheter (Hexacath) and PressureWire X guidewire (Abbott), coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) using bolus thermodilution with the PressureWire X and CFR using Doppler velocity with the ComboWire (Philips). The full protocol is described in Supplementary Appendix 6.
RANDOMISATION PROCEDURE AND BLINDING
Randomisation procedures will occur in 6 high-volume PCI centres in the United Kingdom with a proctor in attendance. Procedures will be performed with standard electrocardiogram and haemodynamic monitoring. A 9 Fr sheath will be implanted into the right internal jugular vein with ultrasound guidance under local anaesthetic. Right atrial pressure will be measured in end-expiration. Participants with a mean right atrial pressure ≥15 mmHg will be withdrawn.
Coronary sinus access will be obtained with a diagnostic catheter, and a coronary sinus venogram will be acquired in the left anterior oblique position to confirm coronary sinus size and anatomy are suitable for implantation.
Auditory isolation will be obtained using over-the-ear headphones playing music. A bolus of 5,000 units of intravenous heparin will be administered. Incremental doses of intravenous benzodiazepines and opiates will be administered to establish a deep level of conscious sedation. Carbon dioxide capnography will be utilised according to local practice. Ventilatory and circulatory status will be monitored throughout the procedure. Once adequately sedated, participants will be randomised 1:1 to CSR or placebo using a dedicated computer randomisation software (Randi; open source software).
In the CSR group, device implantation will be conducted according to standard clinical protocol (Supplementary Appendix 7). During CSR balloon inflation, coronary sinus wedge pressure will be measured where possible. In the placebo group, sedation will be maintained for 15 minutes without further intervention. At the end of the procedure, protamine will be administered, and the venous sheath will be removed under sedation.
A standardised handover to the blinded recovery team will be performed. Participants, caregivers and all staff outside the catheter laboratory, including ward and research staff involved in follow-up assessment and data analysis, will be blinded to treatment allocation. Following ward recovery with routine haemodynamic monitoring, all participants will be discharged with dual antiplatelet therapy for 6 months and standardised discharge documentation. An assessment of the blinding of participants and clinical staff will be made at the time of discharge (Supplementary Appendix 8).
BLINDED FOLLOW-UP EVALUATION
Participants will continue daily completion of the smartphone symptom application and will have access to the blinded research team at any time. Symptom questionnaires, CCS class assessment, treadmill exercise testing and CMR will be repeated at 6 months by a blinded research team.
BLINDING INDEX
Fidelity of the blinding of the patient, clinical and research teams will be assessed and reported prior to discharge after the randomisation procedure and at 6-month follow-up (Supplementary Appendix 8). The blinding index will be assessed using previously reported methodology15.
UNBLINDING AND TRIAL END
After the completion of follow-up and blinding assessment, participants will be unblinded and return to routine care. The placebo group will be offered the opportunity to undergo CSR implantation as part of clinical care. Any actions after unblinding will not contribute to outcome data. To date, 51 patients in ORBITA-COSMIC have been randomised, and 40 have completed follow-up.
DATA MONITORING
ORBITA-COSMIC will be overseen by a trial steering committee with an independent chair. All safety events and participant crossovers will be reported to an independent data safety and monitoring board. Data management is detailed in Supplementary Appendix 9.
STATISTICAL ANALYSIS
The sample size was calculated to detect a change in the between-group difference in myocardial perfusion reserve (MPR) on CMR at follow-up. For simplification, a frequentist approach was used for sample size calculation, as an approximation of the performance of the Bayesian model. The calculation was informed by (1) the only study of perfusion change with the CSR, which was unblinded, single arm, and reported a variable effect, with an 8% difference from baseline to follow-up in the global perfusion and a 35% difference in ischaemic segments5 and (2) a reproducibility standard deviation of MPR of 18%9.
Conservatively estimating the change in ischaemic segments to be 10% with an 18% reproducibility standard deviation, to have a 90% power with a 5% alpha level will require 38 participants. We estimate a crossover and dropout rate of 10% and, therefore, plan to randomise 50 participants.
Categorical variables will be summarised as proportions; continuous variables will be summarised as quartiles. All data will be analysed according to the intention-to-treat principle.
The mechanism of action of the CSR is unknown. The analytical approach is designed to take into consideration previously published concepts: perfusion may improve only in segments which are ischaemic5, normally perfused segments may have reduced perfusion at follow-up3, transmurally infarcted segments will not have reliable perfusion quantification, and ischaemic thresholds in quantified perfusion are arbitrary.
To enable an analysis that can draw multiple simultaneously valid conclusions, a Bayesian modelling approach will be taken. The data from every segment will be used in a model that conditions the stress MBF at follow-up on the stress MBF at enrolment and the treatment arm (CSR or placebo), clustered by participant. An indicator variable, based on whether the segment was classified as ischaemic at enrolment, will also be included as an interaction with the treatment arm.
From this model, the posterior density function for the treatment effect (log-odds ratio [OR]) will be provided, along with the cumulative posterior distribution.
PRIMARY OUTCOME
The primary efficacy analysis will be derived from the model and the effect of the treatment arm within the ischaemic segments. Particular probabilities of OR intervals will be computed, along with 2-sided 0.95 Bayesian credible intervals.
SECONDARY OUTCOMES ESTIMATED FROM THE PRIMARY MODEL
The outcomes of the effect in the designated “non-ischaemic” segments and the effect on global myocardial perfusion will be computed from the same model and presented.
A detailed discussion of the analysis plan for secondary outcomes, including the primary symptom outcome, is included in the statistical analysis plan.
Discussion
The coronary sinus Reducer is recommended in patients with angina, epicardial CAD, no further revascularisation options and optimal antianginal medication16. While the device is hypothesised to redistribute myocardial perfusion to improve ischaemic segments, the mechanism of action of this device is poorly understood and unintuitive, with only single-arm data in humans512. This may explain why, despite placebo-controlled evidence of angina improvement1 and guideline recommendations16, the CSR is not widely used in current clinical practice.
ORBITA-COSMIC is the first placebo-controlled trial to investigate the hypothesised improvement in myocardial perfusion. It incorporates key factors in the design to ensure that assessments are reliable, with the potential to inform clinical practice. Firstly, randomisation will avoid misattribution of regression to the mean to a treatment effect. Secondly, inclusion of a placebo control group will allow quantification of the true physical component of treatment by accounting for the contribution of placebo to the overall effect. Whilst blinding in trials of interventional procedures is complex and challenging, requiring a systemic and reproducible approach, it is certainly possible and important17. It is essential for symptomatic endpoints, where the differences between unblinded and blinded effect sizes are substantial18192021222324. And finally, robust blinding methodology, including auditory isolation, deep levels of conscious sedation, standardised management of blinded clinical and research teams and reporting of blinding fidelity, will ensure that any potential impact of unblinding will be minimised and measured.
To optimise the power of the trial to detect a between-group difference, multiple methodological steps have been incorporated. CMR quantitative perfusion mapping will improve the ability to detect regional changes in myocardial blood flow. Daily collection of patient-reported angina data will increase the ability of the trial to detect change. This contrasts with conventional angina assessment, which is restricted to specific time points with the possibility of recall and reporting biases. Bayesian statistical methodology will be used so that direct inferential statements can be made.
Limitations
The study is only recruiting from centres in the United Kingdom, which may impact the generalisability of the data. Although, these centres serve diverse populations, all efforts will be made to ensure the participants represent the local population. While the study is adequately powered for the primary outcome, the size of this effect will determine our ability to detect changes in the secondary outcomes. Only a proportion of patients will be anatomically suitable to undergo coronary physiology assessment, given the advanced nature of their coronary artery disease.
Conclusions
The CSR is the only interventional procedure for angina relief with a site of action in the cardiac venous circulation. The placebo-controlled mechanism of action and efficacy of the CSR is uncertain. ORBITA-COSMIC will assess the impact of the CSR compared to a placebo procedure on myocardial perfusion and angina in a double-blind, randomised controlled trial.
Impact on daily practice
ORBITA-COSMIC will provide placebo-controlled data on the efficacy of the CSR on myocardial perfusion and angina. The results will inform our daily practice in patients with angina, epicardial CAD, no further options for revascularisation and maximum antianginal medication.
Knowledge of the mechanism of action of the device and its antianginal efficacy will determine if, and when, this device should be offered.
Acknowledgements
We thank Raquel Trujllio, Craig Robertson and Katherine March for their dedication and support.
Funding
The trial is sponsored by Imperial College London and funded by the Medical Research Council (MR/V001620/1). Michael Foley, Christopher Rajkumar and Fiyyaz Ahmed-Jushuf are funded by the Medical Research Council (MR/V001620/1, MR/S021108/1 and MR/W000520/1). James Howard, Rasha Al-Lamee and Darrel Francis are funded by the British Heart Foundation (FS/ICRF/22/26039, FS/ICRF/22/26051 and RG/RG/F/22/110059). The CSR devices used in the trial are funded by a grant from the Imperial Healthcare Charity (Nissen Fund). Graham D. Cole is supported by the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
Conflict of interest statement
M.J. Foley has received speaker honoraria from Menarini and Philips/Volcano. C.A. Rajkumar has received speaker honoraria from Menarini and Philips/Volcano. F. Simader has received financial support from Servier. S. Nijjer has received speaker honoraria from Philips/Volcano, Pfizer, Bayer, AstraZeneca, Ingelheim, and Amarin. R. Petraco has received speaker and consultant fees from Philips and Abbott. J. Howard reports shares in Mycardium AI. G.D. Cole reports shares in Mycardium AI. R. Al-Lamee reports advisory board positions for Janssen Pharmaceuticals, Abbott, and Philips; and speaker honoraria for Abbott, Philips, Medtronic, Servier, Omniprex, and Menarini. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

