Abstract
Aims: Transcatheter mitral valve replacement (TMVR) is a promising therapeutic solution to treat high-risk patients with severe mitral regurgitation (MR) contraindicated to surgery. Optimal selection of patients who will benefit from the procedure is of paramount importance. We aimed to investigate factors associated with TMVR screening.
Methods and results: From November 2016 to July 2018, we examined conditions associated with TMVR screening success in patients referred to the two French heart valve clinics with the greatest TMVR experience. Among a total of 40 consecutively screened patients, 16 (40%) were selected for TMVR (8 Twelve Intrepid, 7 Tendyne and 1 HighLife), while 24 patients (60%) were refused for TMVR mainly because of a too large mitral annulus (MA) (n=15, 62% of those refused), or too small anatomy and risk of neo-left ventricular outflow tract (LVOT) obstruction (n=6, 25% of those refused). Patients with suitable anatomy for TMVR were more often male and more frequently suffered from secondary MR (p=0.01) associated with previous myocardial infarction and presented a commissure-to-commissure diameter less than 39 mm (AUC=0.72, p=0.0085) and LVESD greater than 32 mm (AUC=0.83, p<0.0001) on transthoracic echocardiography, and an MA area less than 17.6 cm² (AUC=0.95, p<0.0001) and anteroposterior diameter greater than 41.6 mm (AUC=0.87, p<0.001) on CT scan.
Conclusions: Despite several prostheses being available, most patients referred to heart valve clinics who are good candidates with regard to their clinical profile cannot have TMVR because of mismatch between their anatomy and prosthesis characteristics. Our findings suggest the need to develop new prostheses adapted to larger mitral annuli but with a lower impact on the LVOT.
Introduction
Mitral regurgitation (MR) is the most prevalent valvular heart disease (VHD) in Western countries, with an age-dependent prevalence and affecting up to 10% of people older than 75 years1,2. Without treatment, severe MR is associated with excess morbidity and mortality and a significant socio-economic impact3,4. Despite these poor outcomes and the undeniable advances in surgical management, only a small proportion of patients suffering from MR undergo surgery, paving the way for percutaneous management strategies5,6.
While several repair techniques have emerged over the last decade, percutaneous mitral valve repair (PMVR) is mostly limited nowadays to the edge-to-edge technique using the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA)7,8,9,10. Nevertheless, some patients are not suitable for this technique because of primary MR with severe calcification, cleft, rheumatic restriction or Barlow’s disease11. Additionally, conflicting data exist on the benefit of treating secondary MR with left ventricular dysfunction12,13.
Recent studies have shown that TMVR using dedicated prostheses is a promising therapeutic solution to treat high-risk patients contraindicated to surgery14,15,16. This technique may offer several advantages considering the complexity and wide variety in the presentation of mitral valve disease. However, the development of TMVR entails several challenges including a careful selection of patients to obtain a sizing adapted to fit the dimensions and the geometry of the mitral annulus (MA) and to minimise the risk of left ventricular outflow tract (LVOT) obstruction17,18.
In the present study, we aimed to investigate the factors associated with TMVR screening failure in the two French heart valve centres allowed to propose TMVR with dedicated prostheses: the Twelve Intrepid™ (Medtronic, Minneapolis, MN, USA), the Tendyne (Abbott Vascular) and the HighLife™ (HighLife, Paris, France) systems.
Methods
STUDY POPULATION AND DESIGN
From November 2016 to July 2018, we prospectively studied all consecutive patients with severe MR referred to the two French heart valve centres (CHU Lille and Clinique Pasteur, Toulouse) for percutaneous implantation because of high risk for MR surgery. Inclusion criteria were: age >18 years with severe (New York Heart Association [NYHA] grade III-IV) and symptomatic (NYHA functional Class ≥II) chronic MR. Patients with severe mitral annulus calcification, left atrial or LV thrombus, prior mitral valve surgery, indicated for standard cardiac surgery or suitable for mitral valve repair using the MitraClip system according to our Heart Team decision were not considered for TMVR screening. The local ethics committees approved the protocol and patients gave informed consent.
ECHOCARDIOGRAPHY
A comprehensive transthoracic (TTE) and transoesophageal echocardiography (TEE) using two- and three-dimensional imaging was performed according to current guidelines19 using state-of-the-art echocardiographic ultrasound systems (Vivid 9 or Vivid 95; GE Healthcare, Little Chalfont, United Kingdom). Special attention was paid to the mechanism, severity and consequences of MR as well as the dimensions and calcification of the MA.
CARDIAC COMPUTED TOMOGRAPHY
Contrast-enhanced cardiac computed tomography (CT) images were also acquired for screening and procedure planning. CT examinations were performed using a multiphase retrospectively electrocardiogram-gated data acquisition. Mitral annular segmentation was performed20 using dedicated software (3mensio; Pie Medical Imaging, Bilthoven, the Netherlands), as previously described. Briefly, mitral annular segmentation was performed at 60% of the cardiac cycle, yielding a D-shaped mitral orifice contour with several parameters including annular area, perimeter, septal-to-lateral and intercommissural diameters.
STATISTICAL ANALYSIS
Continuous variables were tested for normality with the Shapiro test, and are given as mean±SD. Continuous variables with non-Gaussian distribution are given as median (interquartile range [IQR]). Categorical variables are given as percentages of individuals. Patients were separated into three groups according to their anatomy. A too large MA was defined by a compression rate lower than the threshold defined by the prosthesis manufacturer. One-way analysis of variance (ANOVA) was used for comparison of the three groups with a Bonferroni post hoc t-test. Receiver operating characteristic (ROC) curve analysis was used to predict the TTE or CT-scan parameter and cut-off with the highest discriminating power to predict a refusal for too large or too small anatomy. Statistics were performed using MedCalc v16.4 (Ostend, Belgium).
Results
CHARACTERISTICS OF PATIENTS REFERRED TO THE HEART TEAM AND ELIGIBLE FOR TMVR SCREENING
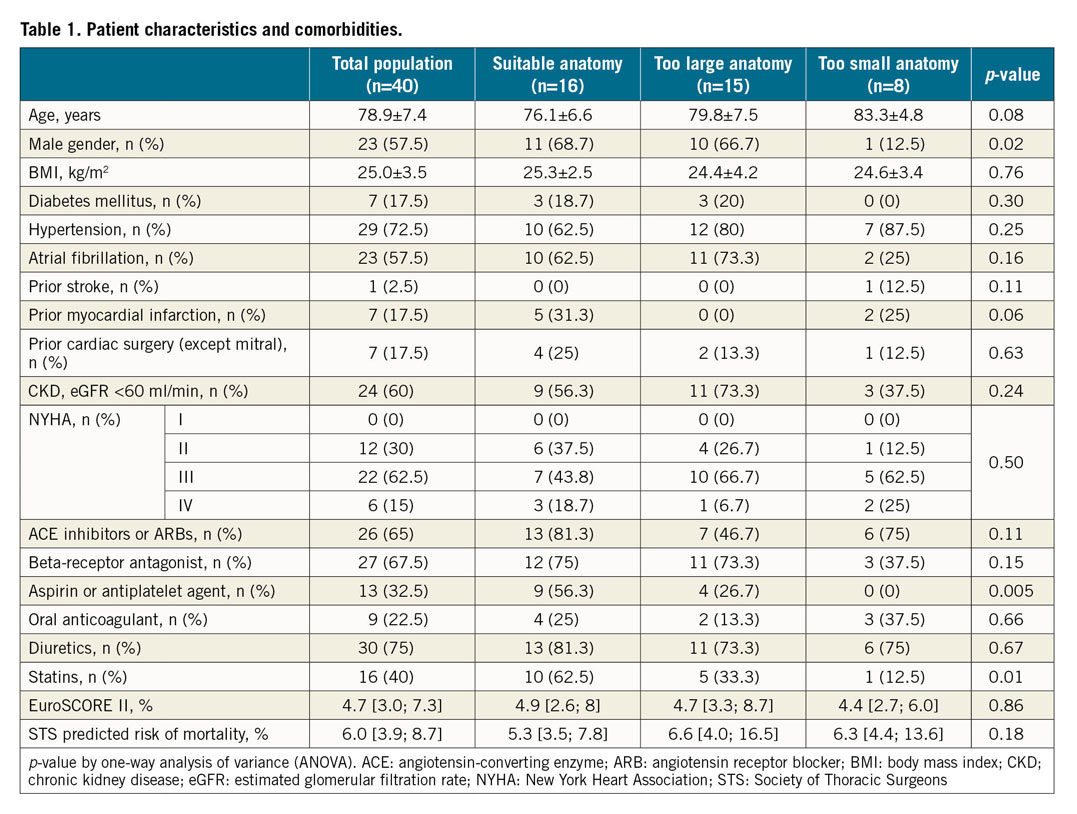
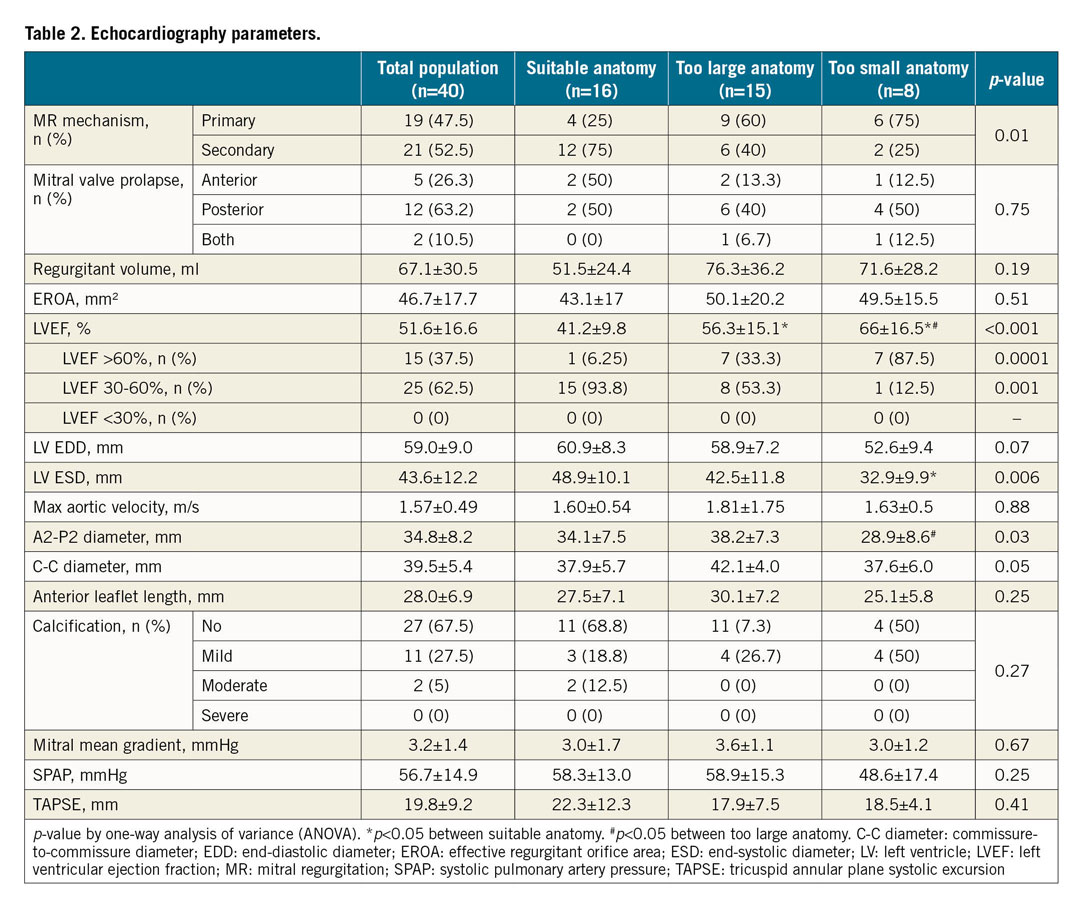
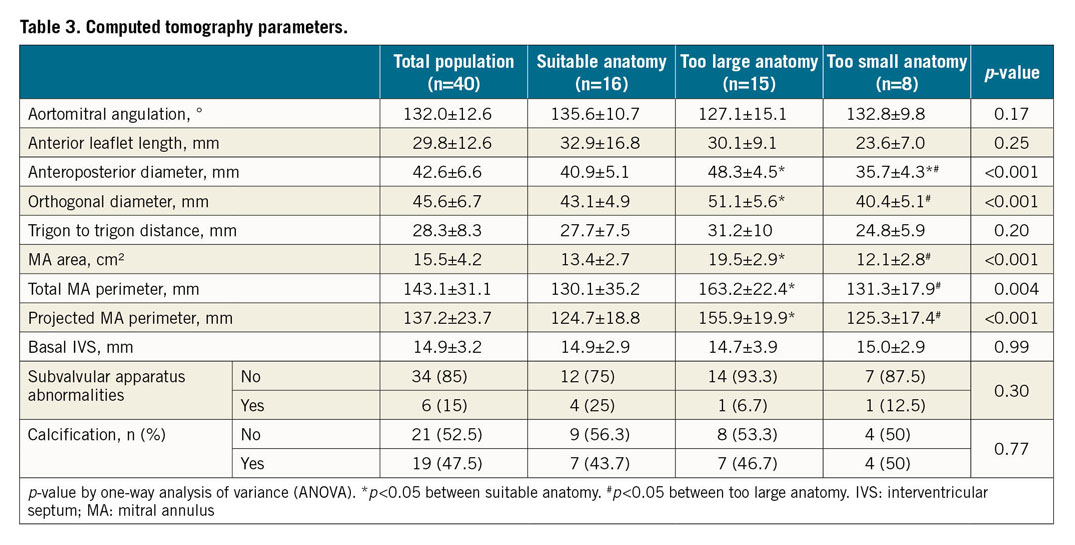
A total of 40 patients were included. Characteristics of the population are summarised in Table 1. Mean age was 79±7 years. The population was 58% male and 18% had diabetes. Most of the patients (70%) were in NYHA Class III or IV. Almost two thirds had chronic kidney disease and 18% had previous cardiac surgery. Median EuroSCORE II and Society of Thoracic Surgeons (STS) predicted risk of mortality were 4.7 (3.0-7.3) and 6 (3.9-8.7), respectively. MR mechanism was mainly secondary (53%) and, in case of primary MR, the posterior mitral valve leaflet was mainly involved (63%) (Table 2). Left ventricular (LV) function was normal (ejection fraction >60%) in 15 patients (37.5%) and moderately impaired (ejection fraction between 30% and 60%) in 25 patients (62.5%). No patient had severe LV dysfunction (ejection fraction <30%). CT analysis showed a mean aorto-mitral angulation of 132±12.6°, an MA area of 15.5±4.2 cm² and a total MA perimeter of 143.1±31.1 mm (Table 3). The anteroposterior and the orthogonal diameter as measured by CT scan was 42.6±6.6 mm and 45.6±6.7 mm, respectively.
SCREENING RESULTS
Among the 40 patients screened, 16 (40%) were selected for TMVR (8 Twelve Intrepid, 7 Tendyne and 1 HighLife), while the remaining 24 patients (60%) were not considered for TMVR, because of too large anatomy (n=15) or too small anatomy (n=8, small annulus diameter [n=2] and/or predicted neo-LVOT obstruction [n=6]) for TMVR (Figure 1, Figure 2, Supplementary Table 1). One patient had too poor LV function and one died during the screening process. The mean time between the Heart Team decision and TMVR was 113±87 days. Examples of screening results are given in Figure 3.
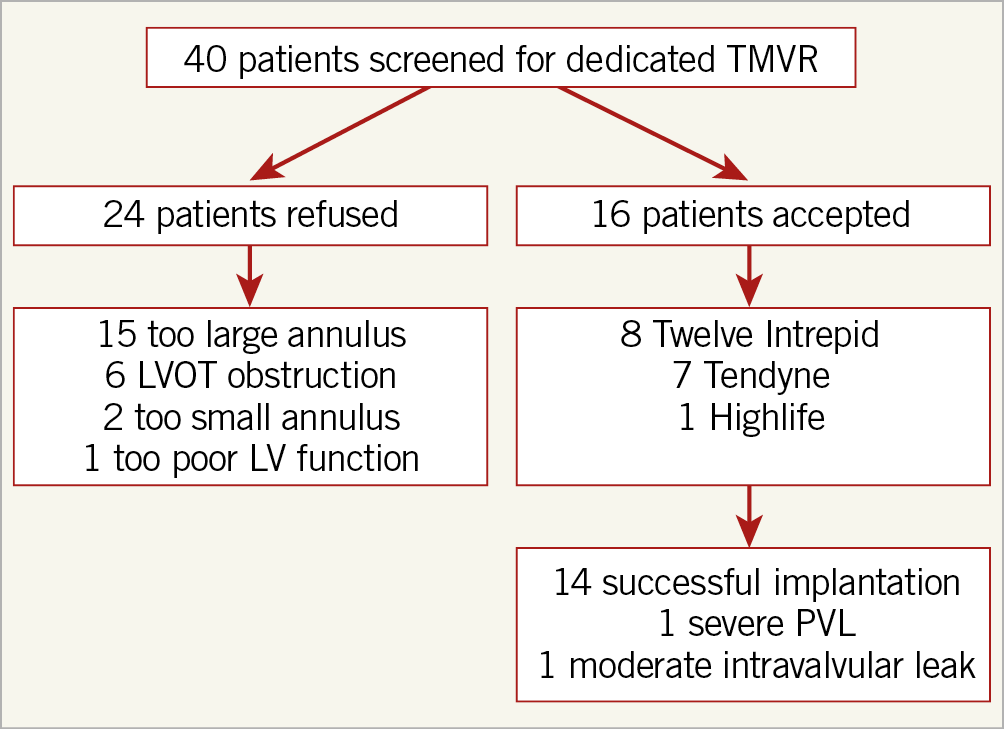
Figure 1. Flow chart.
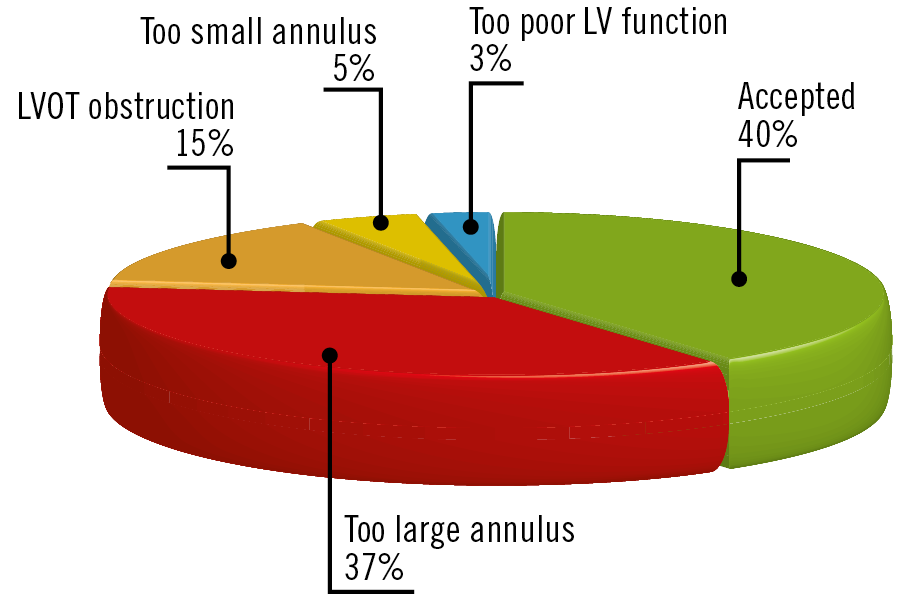
Figure 2. Screening results.
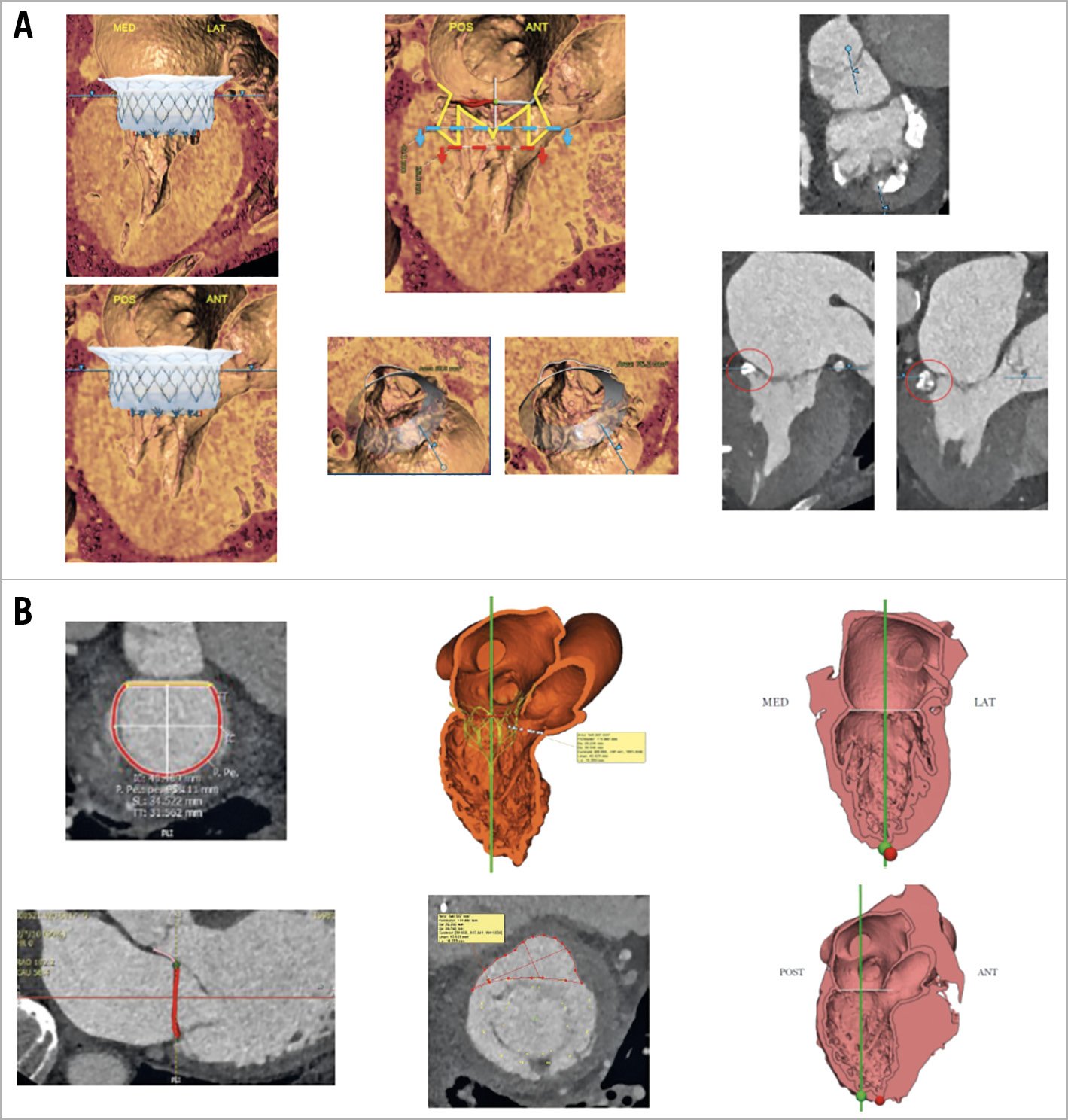
Figure 3. Examples of screening results. A) Screening failure. B) Screening success.
PARAMETERS ACCORDING TO SCREENING RESULTS
Patients with suitable anatomy for TMVR were more often male and suffered more frequently from secondary MR (p=0.01) associated with previous myocardial infarction (p=0.06). They were thus more frequently treated with aspirin or an antiplatelet agent (p=0.005) (Table 1, Table 2). Conversely, patients with either too large or too small anatomy were mainly those with primary MR (75% and 60% vs 25%, p=0.01 respectively). While the severity of the MR was not different, suitable patients for TMVR had greater LV dysfunction (ejection fraction 41±10% vs 56±5% and 66±17%, p<0.0001 respectively) and more enlarged LV (end-systolic diameter 48.9±10.1 mm vs 42.5±11.8 mm and 32.9±9.9 mm, p=0.006 respectively) than patients with either too large or too small anatomy. Regarding the MA dimensions assessed by TTE, A2-P2 and commissure-to-commissure (C-C) diameters logically discriminated the three groups (34.1±5.7 mm vs 38.2±7.3 mm and 28.9±8.6 mm, p=0.03 for A2-P2 diameter, and 37.9±5.7 mm vs 42.1±4.0 mm and 37.6±6.0 mm, p=0.05 for C-C diameter). ROC curve analysis showed that a cut-off of 39 mm for C-C diameter by TTE and of 40 mm for A2-P2 diameter by TTE had the highest discriminating power to predict a refusal for too large anatomy (area under the curve [AUC]=0.72, p=0.0085, and AUC=0.71, p=0.018, respectively) (Supplementary Figure 1A, Supplementary Figure 1B). A cut-off of 32 mm for LV end-systolic diameter (LVESD) was predictive of a refusal for too small anatomy (AUC=0.83, p<0.0001) (Supplementary Figure 1C). The CT parameters to differentiate suitable patients from patients with either too large or too small anatomy were the anteroposterior diameter (40.9±5.1 mm vs 48.3±4.5 mm and 35.7±4.3 mm, p<0.001 respectively), the orthogonal diameter (43.1±4.9 mm vs 51.1±5.6 mm and 40.4±5.1 mm, p<0.001 respectively), the MA area (13.4±2.7 cm² vs 19.5±2.9 cm² and 12.1±2.8 cm², p<0.001 respectively), the total MA perimeter (130.1±35.2 mm vs 163.2±22.4 mm and 131.3±17.9 mm, p=0.004 respectively), and the projected MA perimeter (124.7±18.8 mm vs 155.9±19.9 mm and 125.3±17.4 mm, p<0.001 respectively). ROC curve analysis showed that a cut-off of 17.6 cm² for MA area had the highest discriminating power to predict a refusal for too large anatomy (AUC=0.95, p<0.0001) (Supplementary Figure 2A) and a cut-off of 41.6 mm for the anteroposterior diameter to predict a refusal for too small anatomy (AUC=0.87, p<0.001) (Supplementary Figure 2B). The individual CT parameters of the patients refused for TMVR are given in Supplementary Table 2. ROC curve analyses to predict a too small or a too large anatomy are summarised in Supplementary Table 3.
IMPLANTATION RESULTS
Among the 16 patients who underwent TMVR, 14 patients (87.5%) had successful implantation without complication, 1 (6.25%) presented severe paravalvular leak, and 1 (6.25%) moderate intravalvular leak. After a mean follow-up of 365±287 days, 5 (31%) patients died after TMVR (4 cardiovascular and 1 non-cardiovascular deaths). Among the 24 patients not suitable for TMVR, 4 patients were lost to follow-up, 8 underwent compassionate MitraClip implantation and 5 patients died (25%) (2 after MitraClip).
Discussion
Exploring all consecutive patients referred to high-volume French heart valve clinics during a period of 18 months, we found that i) a majority of patients (60%) were refused for TMVR mainly for too large annulus (62% of refusals) and less frequently for too small anatomy and subsequent risk of neo-LVOT obstruction (25% of refusals), ii) patients with a C-C diameter >39 mm measured by TTE and an MA area >17.6 cm² measured by CT scan were at higher risk of being refused for a too large anatomy, and iii) patients with an LVESD <32 mm measured by TTE and an anteroposterior diameter less than 41.6 mm measured by CT scan were at higher risk of being refused for a too small anatomy.
Controversial results in secondary MR, complexity of the procedure mainly performed with transapical access and anatomical constraints make the development of TMVR more laborious than for transcatheter aortic valve replacement18,21. To date, TMVR is restricted to high-risk and inoperable patients as defined by the Heart Team, and the experience of TMVR is still very limited, with a few hundred patients included in feasibility studies14,15,16.
Decreasing the time between the decision of the Heart Team and TMVR (four months in our study) is warranted to suit a target population that requires a rapid decision, i.e., frail patients exposed to frequent cardiac events such as death and hospitalisation for recurrent heart failure. A better understanding of screening failure reasons in real-life patients will avoid screening many patients in vain. We thus propose C-C diameter and LVESD measured by TTE as relevant first-line parameters in order to avoid further tedious and time-consuming CT scans. The threshold values we showed should be tested in further studies.
Our rate of screen failure is in line with previous studies with the Tendyne14 and Twelve systems15, reporting a rate of 60% and 70%, respectively. The main anatomical reason for refusal was a too large MA (62% of refusals) in patients probably at the end stage of their mitral disease with significant LV and atrial dilatation. The second major concern with screening was small anatomy with a risk of obstruction of the LVOT. Of note, the evaluation of the risk of LVOT obstruction is challenging as a result of the D or saddle shape of the MA and its dynamic variation during the cardiac cycle22. A dynamic evaluation of the mitral annulus will probably be useful to assess the real impact of the prosthesis on the LVOT better.
In the future, since there is currently a mismatch between the anatomical reality of the candidate patients and the size and the clutter of existing prostheses, the propagation of TMVR implantation will depend on the design of the prostheses. Our findings suggest the need to develop new prostheses adapted to larger MA but with lower impact on the LVOT.
Limitations
The small number of our population is the main limitation. Our findings should be validated and confirmed in larger clinical trials. The final suitability decision was given by the manufacturer leading the feasibility study. The criteria for suitability were the same for the three devices. However, these criteria must be confirmed in the future according to each centre’s experience. Moreover, patients with Barlow’s disease were deemed inappropriate for MitraClip implantation. This relative contraindication must be confirmed for recent MitraClip innovations (MitraClip XTR). Finally, given the small size of our population and the short period of follow-up, it is not possible to draw any conclusion regarding outcomes.
Conclusions
Despite several prostheses being available, most patients referred to heart valve clinics and who are good candidates regarding their clinical profile cannot have TMVR mainly because of a too large annulus. C-C diameter and LVESD measured by TTE and MA area measured by CT scan could be proposed as first-line criteria in the pre-selection process to avoid unnecessary screening. Our findings suggest the need to develop new prostheses adapted to larger MA but with lower impact on the LVOT.
|
Impact on daily practice C-C diameter and LVESD measured by TTE and MA area measured by CT scan could be proposed as first-line criteria in the pre-selection process to avoid unnecessary screening for TMVR. |
Conflict of interest statement
N. Dumonteil is a consultant for Abbott Vascular, Boston Scientific, Edwards Lifesciences and Medtronic. T. Modine is a consultant for Abbott Vascular and Medtronic. The other authors have no conflicts of interest to declare.

