
Abstract
Aims: The high frequency of screening failure for anatomical reasons in patients with severe mitral valve regurgitation (MR) is a limiting factor in the screening process for transcatheter mitral valve replacement (TMVR). However, data on optimal patient selection are scarce. The present study aimed to develop a screening algorithm based on TMVR screening data.
Methods and results: A total of 195 screenings for six different TMVR devices were performed in 94 high-risk patients with severe MR. We compared baseline echocardiographic and multislice computed tomography (MSCT) parameters between the subgroups of patients accepted (N=33) and rejected for TMVR (N=61). Reasons for screening failure were assessed, and a decision tree algorithm was statistically derived. Reasons for screening failure were small LV dimensions (30.6%), small (7.5%) or large (22.5%) annular size, potential risk of LVOT obstruction (22.0%) or mitral annulus calcification (15.6%). A four-step decision tree algorithm to assess TMVR eligibility was developed resulting in an AUC of 0.80 (95% CI: 0.71, 0.89, p<0.0001).
Conclusions: This study presents the first screening algorithm to assess anatomical eligibility for TMVR in patients with severe MR, based on simple MSCT criteria. Given the high rate of TMVR screening failure, this algorithm may facilitate the identification of potential TMVR candidates.
Introduction
Transcatheter mitral valve replacement (TMVR) represents a complementary therapeutic approach for surgical high-risk patients with severe mitral valve regurgitation (MR). This novel therapy promises to reduce MR as durably as surgical valve replacement while reducing the procedural risk with an interventional approach1. Given the complex structure of the mitral valve apparatus and possible interactions of an implanted device with anatomic structures, careful patient selection by echocardiography and multislice computed tomography (MSCT) screening is required2,3. ECG-gated cardiac MSCT enables comprehensive 3D volumetric assessment by providing an exact characterisation of the anatomy of the subvalvular apparatus and the geometry of the mitral valve4.
During the screening process, a majority of patients is deemed ineligible for TMVR due to clinical or anatomical reasons, resulting in a high rate of screening failure5,6,7. An evidence-based selection algorithm may facilitate and improve decision making in the screening process for TMVR1. This study aimed to create a statistically derived decision tree based on anatomical screening data, enabling simple and reliable identification of potential TMVR candidates.
Methods
STUDY POPULATION
From 2016 to 2019 a total of 94 high-risk patients with severe MR who were considered ineligible for surgery or endovascular edge-to-edge repair underwent screening for TMVR at our centre. Anatomical eligibility was assessed on the basis of MSCT and echocardiographic patient data. Patients were screened for six different dedicated transapical or transseptal TMVR devices. Devices and numbers of screened patients per device are given in Supplementary Table 1. Patients not clinically suitable for TMVR were excluded from the study beforehand. All patients provided written informed consent for device screening and data acquisition.
Detailed information on the TMVR screening process is given in Supplementary Appendix 1.
ECHOCARDIOGRAPHIC ANALYSES
Transthoracic and transoesophageal echocardiography were performed in every patient for assessment of MR severity and aetiology as well as functional and morphological status. Evaluation of MR was performed according to the 2017 ESC/EACTS guidelines for the management of valvular heart disease8. Mean mitral gradient effective regurgitant orifice area (EROA), vena contracta diameter, mitral regurgitation volume and systolic pulmonary artery pressure were documented according to a standardised protocol. Furthermore, left ventricle (LV) characterising parameters were assessed including LV ejection fraction (EF), LV end-diastolic diameter (LVEDD) and volume (LVEDV) as well as LV end-systolic diameter (LVESD) and volume (LVESV).
CARDIAC MSCT ANALYSES
ECG-gated full cardiac cycle MSCT was performed in every patient who underwent the screening process. Subsequently, a dedicated software (3mensio Structural Heart V9.1; Pie Medical Imaging, Maastricht, the Netherlands) was used to assess the mitral valve complex, LV dimensions, LVOT and aortomitral continuity at 30% (end-systole) and 75% (mid-to-end-diastole) of the cardiac cycle, as suggested previously4. Mitral valve annulus was sized according to the D-shaped annulus concept that has been described previously9. Accordingly, mitral valve annulus perimeter and area were assessed as well as septal-lateral (SL) diameter, intercommissural (IC) diameter and intertrigonal (TT) distance (Figure 1, Figure 2),10. Mean mitral annulus diameter (Dmean) was calculated according to Abdelghani et al: [(IC diameter + SL diameter) / 2]11. In addition, MSCT analyses comprised LV dimensions (annulus-to-apex [ATA] distance) (Figure 1A, Figure 2A), aortomitral angulation, distances to anterolateral and posteromedial papillary muscles and the extent of mitral annulus calcification (MAC). Native left ventricular outflow tract (LVOT) area was standardly measured at 5 mm below the aortic valve annulus in end-systole.
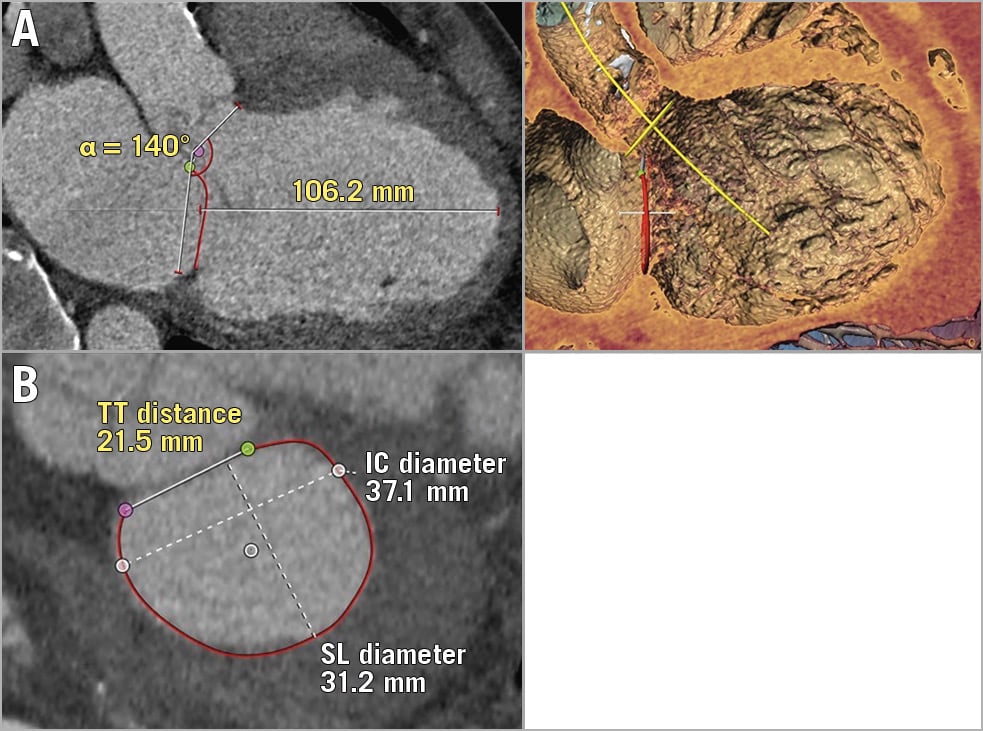
Figure 1. Favourable TMVR anatomy (example). MSCT reconstruction via 3mensio Structural Heart V9.1. A) End-diastolic 2D (left) and 3D (right) three-chamber view with flat aortomitral angle and large annulus-to-apex (ATA) distance. B) Measurements of D-shaped mitral valve annulus including intertrigonal (TT) distance, intercommissural (IC) diameter and septal-lateral (SL) diameter.
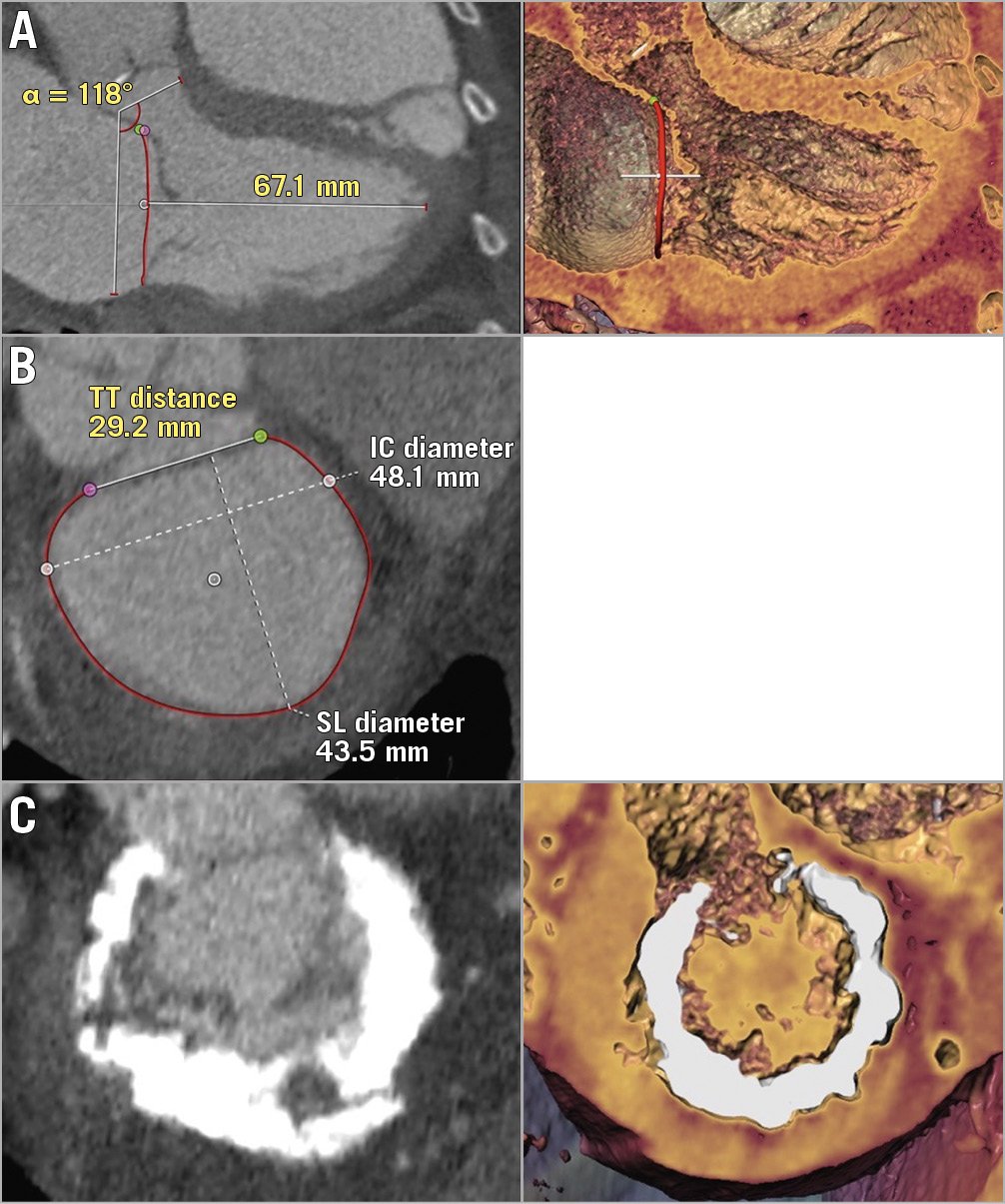
Figure 2. Unfavourable TMVR anatomies (examples). A) End-diastolic 2D (left) and 3D (right) three-chamber views with acute aortomitral angle, small native LVOT and small ATA distance. B) Measurements of a large mitral valve annulus including TT distance, IC diameter and SL diameter C) Circumferential mitral annulus calcification.
STATISTICAL ANALYSIS
Patients (N=94) were stratified according to acceptance for a TMVR device (accepted group; n=33, 35.1%) or ultimate rejection for all devices screened for (rejected group; n=61, 64.9%). We considered echocardiography and MSCT variables as potential predictors of an eligible or an ineligible TMVR anatomy. We excluded variables with more than 30% missing values and variables that were used to calculate other variables among the set of potential predictors. Eventually, 15 echocardiography and MSCT variables were left as input for the derivation of the decision tree algorithm (Supplementary Table 2). KNIME version 3.7.2 was used to derive a decision tree with the Gini index as quality measure and pruning based on minimal description length. A detailed description of the statistical derivation process is given in Supplementary Appendix 2.
Results
Clinical baseline characteristics of all patients and of the subgroups (accepted and rejected groups) are presented in Supplementary Table 3.
ECHOCARDIOGRAPHIC BASELINE CHARACTERISTICS
Echocardiographic parameters at baseline are shown in Table 1. The aetiology of MR was functional in 52.1% (n=49), degenerative in 38.3% (n=36) and of mixed aetiology in 9.6% (n=9) in all screened patients. FMR was present more often in patients accepted for TMVR, whereas rejected patients more frequently suffered from DMR than accepted patients. Accordingly, patients in the accepted group had significantly lower baseline EF and larger LVESV. Moderate or severe tricuspid regurgitation (TR) was prevalent in more than half of all screened patients. However, there were no statistically significant differences between accepted and rejected patients regarding concomitant heart valve disease. No other differences were observed regarding all other echocardiographic parameters.
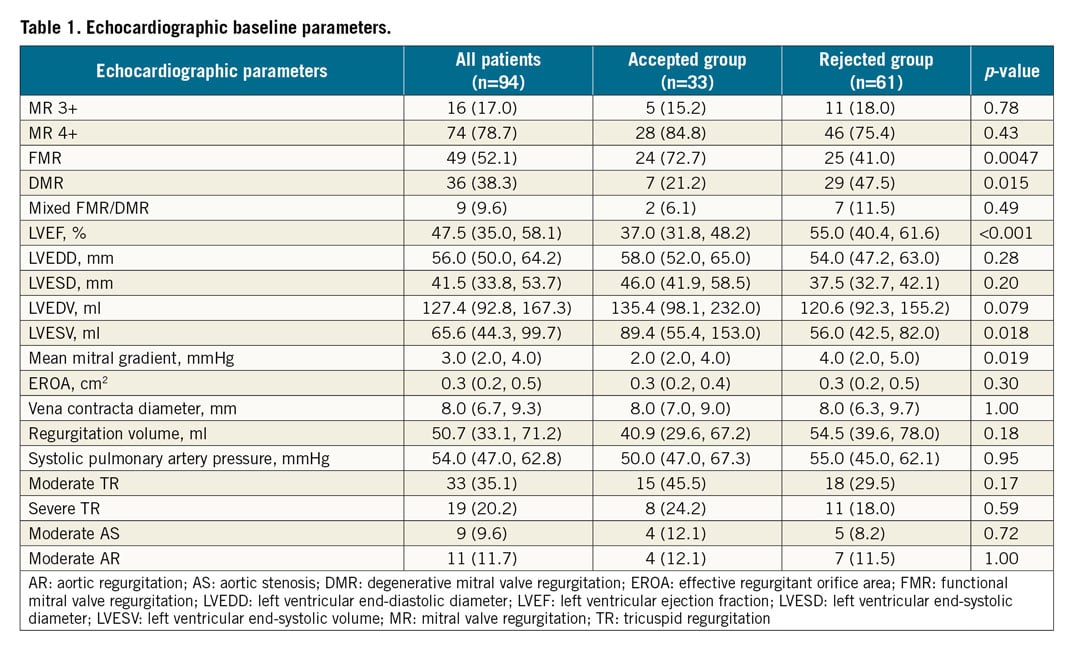
MSCT BASELINE CHARACTERISTICS
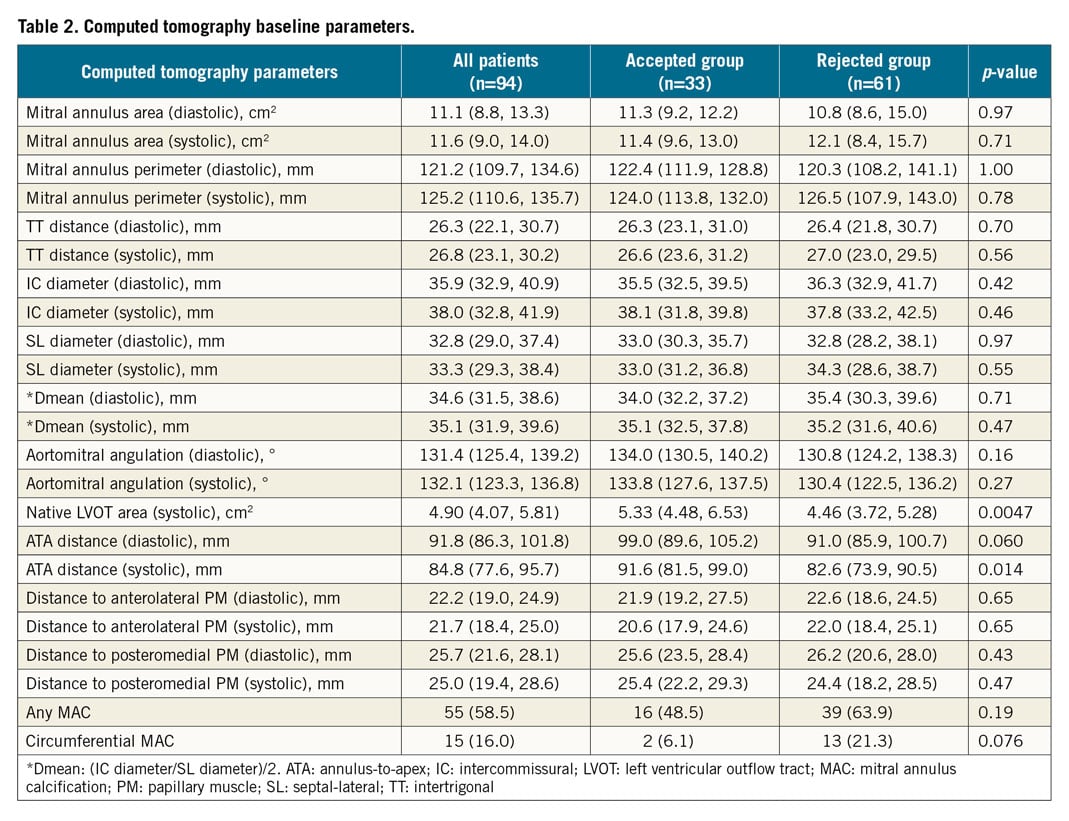
MSCT baseline parameters are shown in detail in Table 2. LV size was significantly different in MSCT measured by systolic ATA distance. Native LVOT area was larger in the accepted group than in the rejected group and circumferential MAC was more frequent in the rejected group. However, the disparity in MAC between accepted and rejected patients did not reach statistical significance. There were no significant differences between the subgroups for all other MSCT parameters.
SCREENING RESULTS AND REASONS FOR SCREENING FAILURE
From a total of 94 patients screened for different TMVR devices, 33 patients were accepted for TMVR device deployment, whereas 61 patients were ultimately rejected for implantation of each screened device. The majority of patients underwent screening for more than one TMVR device (n=62, 66.0%), resulting in a total number of 195 device screenings performed. The screening failure rate was 83.1% (Figure 3). Supplementary Table 4 summarises the screening decisions for all patients and all screened devices, including reasons for screening failure. According to the statements communicated by device manufacturers, five reasons for TMVR screening failure were identified. Spatial restraints of LV dimensions led to the rejection of patients in 30.6%. Mitral annular size was too small in 7.5% and too large in 22.5% of failed screenings. The anticipated risk of LVOT obstruction and MAC were prohibitive conditions in 22.0% and 15.6%, respectively. Maxima and minima of selected baseline MSCT and echocardiography parameters in the accepted group are presented in Supplementary Table 5.
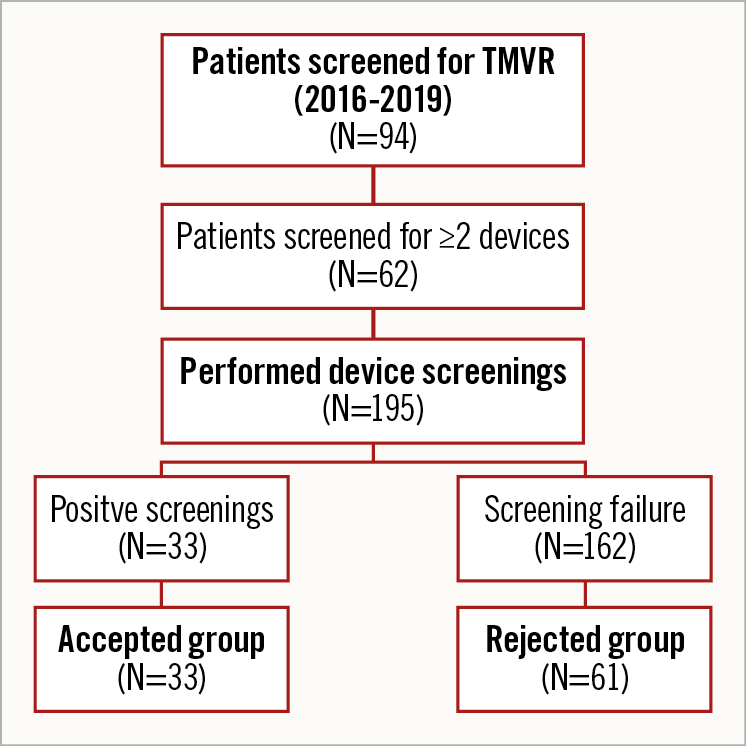
Figure 3. Flow chart of TMVR screenings performed.
Out of 33 patients formally accepted for device deployment, 27 patients eventually underwent TMVR. Three patients died before the planned date of the procedure, two patients withdrew their consent and one patient moved to another city.
DECISION TREE ALGORITHM
A decision tree algorithm was derived taking into account all available baseline MSCT and echocardiographic parameters as well as the results of all TMVR screenings performed (n=195). The best performing decision tree algorithm comprised four essential baseline MSCT parameters leading to either an eligible or an ineligible TMVR anatomy (Figure 4). Of note, baseline echocardiographic parameters were not selected by this algorithm. Stepwise decisions were made on the basis of systolic mitral annulus area (cut-off 8.6 cm2), systolic Dmean (cut-off 38.3 mm), aortomitral angulation (cut-off 130°) and ATA distance (cut-off 100 mm). The algorithm predicted the correct decision (eligibility or ineligibility for TMVR) in 81.9% (n=77) of all patients. Nine patients were falsely excluded by the algorithm – two patients for too small an annulus (area ≤8.6 cm2), six patients for too large an annulus (Dmean >38.3 mm) and one patient for the combination of sharp aortomitral angulation (≤130°) and small LV dimensions (ATA distance ≤100 mm). Conversely, eight patients were incorrectly labelled eligible for TMVR. These discrepancies between observed and predicted decisions resulted in a positive predictive value (PPV) of 75.0% (95% CI: 56.6, 88.5) and a negative predictive value (NPV) of 85.5% (95% CI: 74.2, 93.1). The area under the curve (AUC) for all decisions of the proposed algorithm was 0.80 (95% CI: 0.71, 0.89; p<0.0001) (Figure 5).
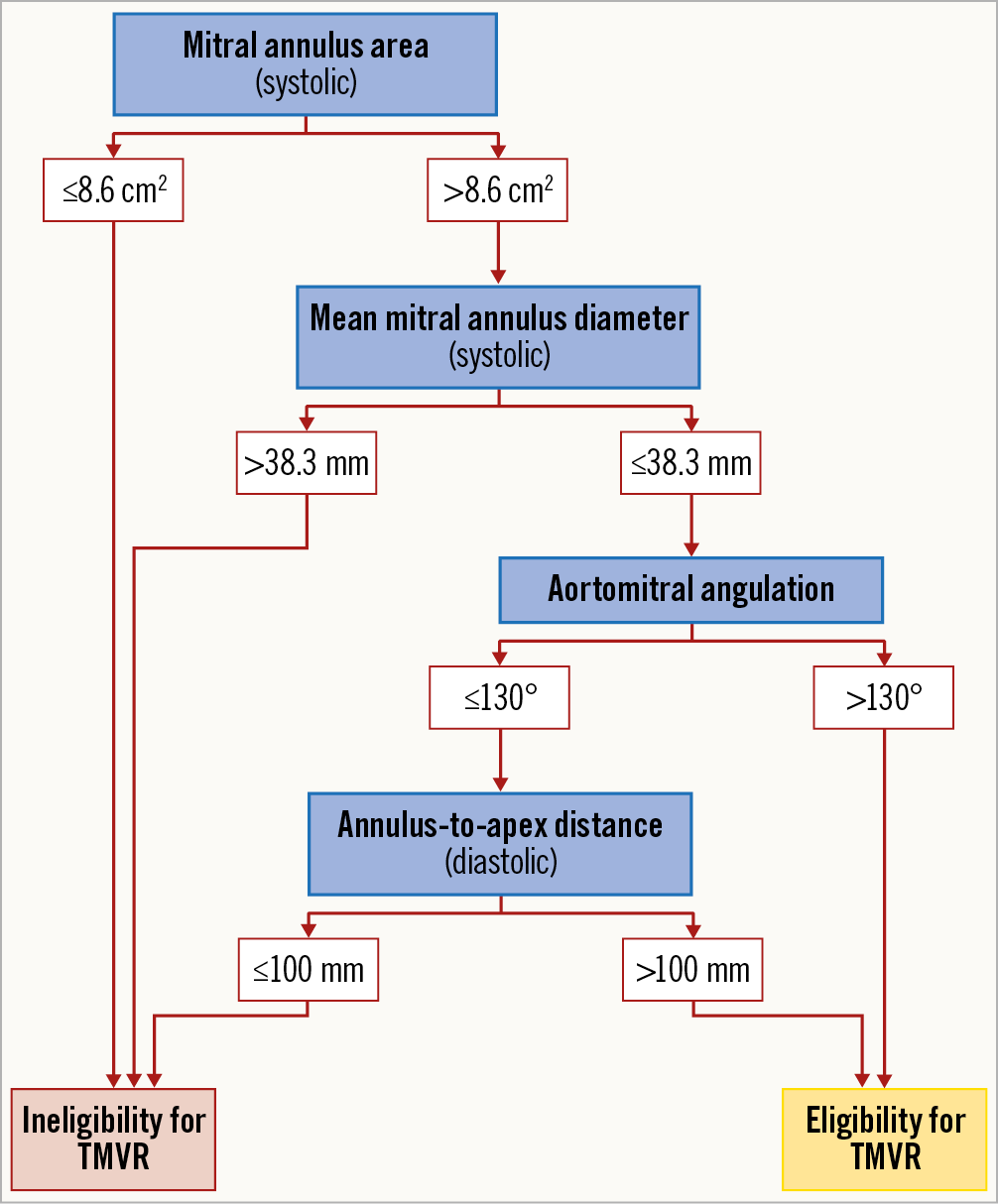
Figure 4. Decision tree screening algorithm.
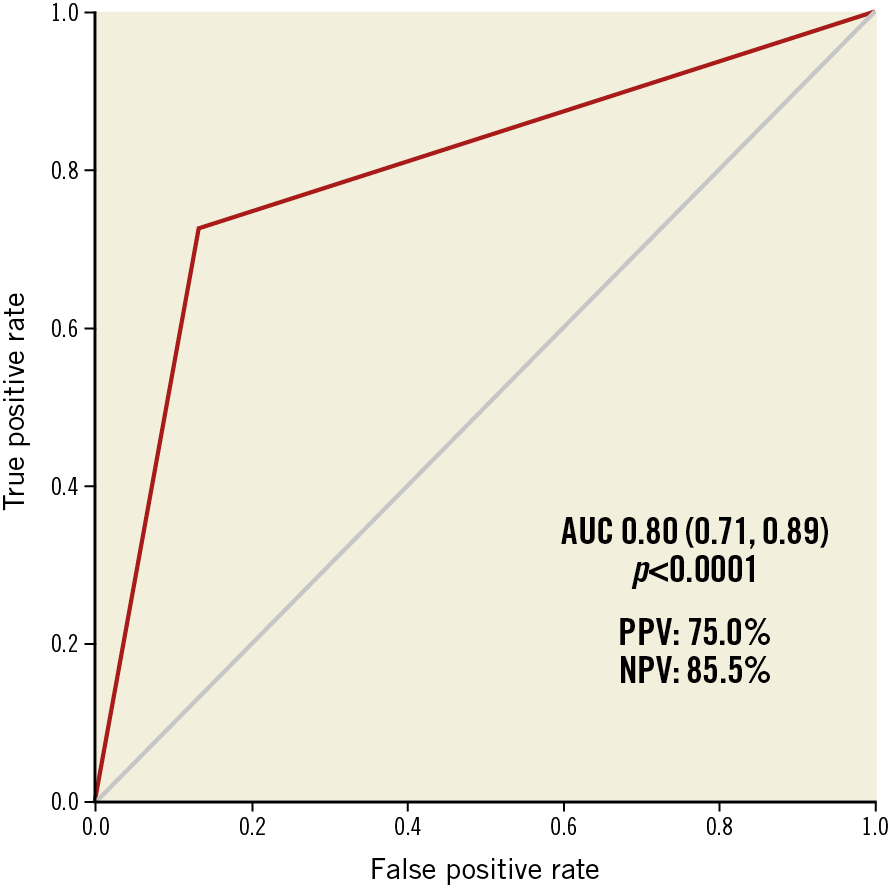
Figure 5. Statistical performance of the screening algorithm.
Discussion
This study reports an analysis of the largest single-centre screening experience of patients with severe MR for TMVR to date. Based on a total of 195 device screenings performed in 94 patients across multiple device platforms, we identified common reasons for screening failure and derived a screening algorithm, based solely on MSCT parameters, enabling an accurate identification of MR patients anatomically eligible for TMVR.
Based on the patient population and the device selection available for this study, an “optimal TMVR anatomy” can be defined as follows. First, the mitral annulus size must lie within the size range of the specific device in question. Although the proposed algorithm suggests upper and lower size limits, an up-to-date annular size range should be derived from companies’ sizing charts and is highly variable due to the development of new devices. However, our algorithm can help to identify patients with a lower probability of a positive TMVR screening result due to sizing issues.
Second, in order to avoid interference of the valve with LV myocardium, FMR patients seem to represent a preferred target group as LV dimensions are mostly larger in this patient subset compared to DMR patients. This restriction might be softened as new device shapes might focus on reduction of overall device size. In fact, the development of smaller devices will be a key factor for opening TMVR treatment towards a much larger patient population including DMR patients. This is also of particular clinical relevance as a substantial proportion of DMR patients with certain structural leaflet abnormalities (e.g., clefts, very large coaptation defects, Barlow’s disease, etc.) are unsuitable for currently available percutaneous mitral valve repair devices and – in cases with high surgical risk – are often left untreated12.
Third, the risk of LVOT obstruction has to be taken into account. A haemodynamically relevant LVOT obstruction after implantation of the device might have fatal consequences and has to be ruled out prior to TMVR device deployment. A flat aortomitral angle and a large native LVOT play complementary roles in the identification of a favourable anatomy with low risk of LVOT obstruction. A parallel orientation of mitral and aortic annular planes is associated with a low risk, whereas a perpendicular orientation confers a high risk of LVOT obstruction13. Regarding individual device geometry, prediction of the minimal cross-sectional area of the neo-LVOT in MSCT should be considered, if virtual valve simulation is accessible14,15. A neo-LVOT area of ≤2.0 cm2 is a commonly accepted minimal cut-off for predicted LVOT obstruction15. Accordingly, recent studies retrospectively investigating MSCT data prior to TMVR in degenerated mitral bioprostheses, failed mitral annuloplasty rings or extensive MAC by using non-dedicated transcatheter aortic valve implantation devices found that neo-LVOT areas <1.7-1.9 cm2 were associated with a high risk for haemodynamically relevant LVOT obstruction16,17. However, LVOT obstruction is a physiological phenomenon that remains difficult to predict because it depends on multiple, additional factors that have to be considered, e.g., LV size, length of the anterior mitral valve leaflet or diastolic LV function. Moreover, differences in device shape or protrusion of the device into the LV might also have an impact on LVOT obstruction. In this regard, use of TMVR systems with a supra-annular design appears to be an interesting concept that might overcome the limitations of both LV size and LVOT obstruction18. However, experience with these devices is limited and further investigation is necessary.
Fourth, although TMVR can be performed in the presence of excessive MAC, it certainly is not a favourable condition as there is a potential risk for incomplete sealing and device unfolding as well as for paravalvular leakage. Initially, severe MAC represented an exclusion criterion for all emerging TMVR studies. Uncertainties about the feasibility of performing TMVR in severe MAC might have contributed to aggressive patient exclusion from device deployment in the early phase of patient recruitment for all TMVR devices. Based on growing clinical experience with TMVR in severe MAC, it is not generally considered a contraindication for TMVR anymore, and patients with MAC have in fact been treated successfully with TMVR19. Accordingly, MAC is not included in the presented algorithm, reflecting a less important role of MAC in screening for TMVR eligibility.
Summarising these criteria, as an example, Figure 1 demonstrates a favourable TMVR anatomy, as assessed by MSCT, with large LV dimensions, a flat aortomitral angle and a large native LVOT. Conversely, Figure 2A and Figure 2C show MSCT anatomies of different patients who were rejected because of small LV size and predicted neo-LVOT obstruction (Figure 2A), large mitral annular size (Figure 2B) or circumferential MAC (Figure 2C).
The presented decision tree algorithm (Figure 4) comprises all relevant parameters that should be considered in the evaluation of a patient’s anatomy in the screening for TMVR. Initially, it identifies a lower and an upper limit for eligible annular dimensions, excluding all patients suggestive of very small or very large annuli. Furthermore, it considers the risk of LVOT obstruction by defining a minimum aortomitral angle. The final step of the presented decision tree considers LV size which is represented by diastolic ATA distance. Echocardiographic parameters were statistically outperformed by MSCT parameters. Hence, standard variables such as LVEDD or LVESD were not selected, whereas ATA (Figure 1A, Figure 2A), a less frequently used longitudinal MSCT parameter, plays a decisive role in the algorithm.
Recently, Coisne et al published a study investigating factors associated with TMVR screening failure and success in a series of 40 patients. In line with our results, the authors found a high rate of screening failure and similar reasons for ineligibility. The authors further tested single parameters regarding their ability to predict screening failure. Compared to our results, the assessed cut-offs were considerably higher, reflecting larger annuli in an overall smaller group of investigated patients20.
Despite the high precision of the presented algorithm, we recommend thorough screening for TMVR eligibility regarding all relevant factors of an optimal TMVR anatomy assessed by a multimodality approach, as described above. Future analyses will have to evaluate whether a screening algorithm predicting anatomical eligibility is also able to predict favourable procedural outcome.
The most relevant value of the proposed algorithm might be the achievement of quicker decision making and the avoidance of vain screening of patients definitely not eligible for any TMVR therapy. Instead, these patients should undergo early evaluation of other therapeutic options (e.g., intensification of medical therapy, edge-to-edge repair with supposedly suboptimal outcome, percutaneous annuloplasty, high-risk surgery).
Limitations
Our study has several inherent limitations. The data presented are the result of a retrospective statistical attempt to reflect the given screening data with the best performing algorithm. The algorithm might need slight adaptation and continuous updating to reflect newly emerging devices or changes in existing device platforms. Moreover, the applicability of the algorithm might be hampered by the underlying study design which included multiple TMVR devices and, thus, potentially neglected device-related factors for unsuitability. Further, the design as a single-centre experience might have generated bias in screening processes and choice of device. Especially in the early phase of TMVR screening, the decision about the first device screening was arbitrary and based mainly on the availability of devices. Lastly, both pre-screening of patients included in the analysis and an aggressive patient exclusion in the initial phase of patient selection might have biased our results to a certain extent.
Conclusions
This study attempts for the first time to derive an anatomical algorithm for the screening of patients with severe MR for TMVR. The algorithm is designed as a four-step decision tree based on simple MSCT parameters and might help to identify potential TMVR candidates. Validation of the presented algorithm in a multicentre patient cohort is required to prove its universal applicability.
|
Impact on daily practice Based on screening data across multiple device platforms, we provide unique information on an optimal TMVR anatomy. In addition, for the first time, we present a TMVR screening algorithm that might notably contribute to an improved identification of possible TMVR candidates and a reduced occurrence of TMVR screening failure. |
Acknowledgements
We thank Roya Sedighian for her efforts in data acquisition.
Conflict of interest statement
S. Ludwig reports travel compensation from Edwards Lifesciences, outside the submitted work. F. Deuschl reports personal fees and non-financial support from Neovasc, Edwards Lifesciences, Abbott and Polares Medical, and is currently a full-time employee with Edwards Lifesciences, involved in tricuspid therapies. N. Schofer reports personal fees from Boston Scientific, and travel compensation from Abbott and Edwards Lifesciences, outside the submitted work. D. Kalbacher reports personal fees from Abbott Vascular and Edwards Lifesciences, outside the submitted work. S. Blankenberg reports grants and personal fees from Abbott Diagnostics, Bayer and Thermo Fisher, grants from Siemens and Singulex, and personal fees from Abbott, AstraZeneca, Amgen, Medtronic, Pfizer, Roche, Novartis and Siemens Diagnostics, all outside the submitted work. L. Conradi received lecture fees from and was a proctor as well as an advisory board member of Abbott and Neovasc during the conduct of the study. E. Lubos reports grants and personal fees from Abbott Vascular and personal fees from Edwards Lifesciences, during the conduct of the study, and personal fees from Abiomed, AstraZeneca, Bayer, New Valve Technology and Novartis, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

