Introduction
The early concept development of transcatheter aortic valve replacement (TAVR) paralleled that of percutaneous mitral repair approaches1. Despite a similar timeline for the early work for both aortic and mitral technologies, the rapid pace of development of TAVR has clearly overtaken the development of percutaneous mitral repair. The relative complexity of both the mitral valve apparatus and the spectrum of pathology producing mitral regurgitation (MR) is largely responsible for this more complicated and slower development.
The MitraClip experience
A wide range of percutaneous mitral repair technologies has been described, but to date only two have been used in significant numbers of patients. Leaflet repair with the MitraClip (Abbott Vascular, Santa Clara, CA, USA) and coronary sinus annuloplasty with the CARILLON® Mitral Contour SystemTM (Cardiac Dimensions, Inc., Kirkland, WA, USA) both have CE mark approval and are being used commercially. Several other devices are in trials, and several have fallen by the wayside and are no longer under development2.
The largest experience in human use for percutaneous treatment of MR is with the MitraClip3. To date, almost 9,000 patients have been treated worldwide. The device was first implanted in a patient in 2003 and CE mark approval was achieved in 2008. There has been a steady accumulation of clinical experience since this initial use (Figure 1). The largest single trial utilising this device was the EVEREST II trial4. This was a randomised comparison of the MitraClip device with surgical therapy utilising either conventional valve replacement or repair. Two hundred and seventy-nine patients were included in the trial and randomised in a 2:1 ratio. At 12 months, the rates of the primary endpoint for efficacy were 55% in the percutaneous repair group and 73% in the surgery group (p=0.007).
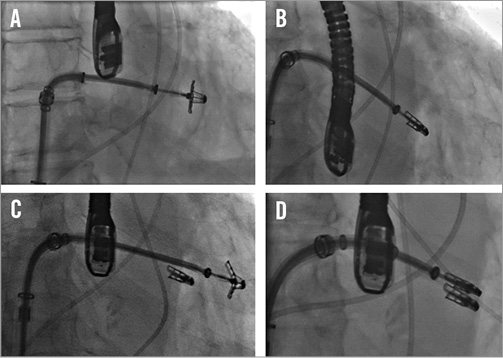
Figure 1. Fluoroscopic images of a MitraClip procedure. A) A MitraClip device has been passed across the mitral leaflets. B) The clip has been pulled back to grasp the leaflets. C) A second clip is passed adjacent to the first. Two clips are used in 40% of cases. D) The second clip has grasped the leaflets and been closed35.
The respective rates of the components of the primary endpoint were as follows: death, 6% in each group; surgery for mitral valve dysfunction, 20% versus 2%; and grade 3+ or 4+ MR, 21% versus 20%. Major adverse events occurred in 15% of patients in the percutaneous repair group and 48% of patients in the surgery group at 30 days (p<0.001). At 12 months, both groups had improved left ventricular size, New York Heart Association (NYHA) functional class, and quality-of-life measures, as compared with baseline. The major conclusion of this study was that the MitraClip is less effective than surgery at reducing MR, but results in similar clinical outcomes and is safer.
About 20% of MitraClip patients underwent mitral surgery within the first six months after treatment, but then MitraClip-treated patients had similar event rates over the next few years (Figure 2). About half the failures in the first six months were due to loss of the insertion of one leaflet into the clip, “partial leaflet attachment”. This failure mode occurred in almost 10% of cases in the early EVEREST II experience, but has subsequently decreased to about 1% of cases. The acute success rate has similarly increased from 85% to over 95%.
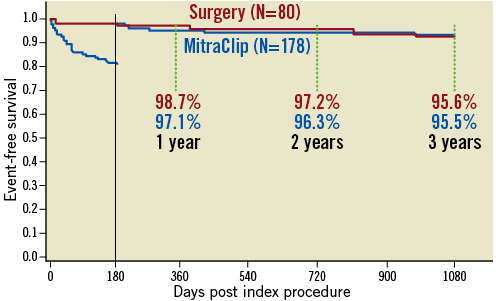
Figure 2. Freedom from mitral valve surgery in MitraClip group or reoperation in surgery group from the EVEREST II trial. Almost all patients who required reoperation in the MitraClip-treated group did so within the first six months after the index treatment. About half of these were due to persistent MR, and the remainder were associated with partial leaflet attachment of the device. After six months there were no differences in the reoperation rate when MitraClip therapy was compared to surgical valve repair or replacement38.
Subgroup analysis of the patients in the EVEREST II trial showed that the procedure had an overall outcome closest to surgery among patients who were older ( ≥70 years old), had abnormal left ventricular function (ejection fraction <60%), and had functional MR. Accordingly, there has been a great deal of study of patients fitting this latter category of older age, poor ventricular function, and functional MR. These patients are typically high risk for surgery and usually do not undergo surgical therapy for MR. Many series have reported excellent clinical outcomes in this group, with symptomatic and clinical improvement despite some residual MR, and a remarkable degree of safety for the procedure. Outcomes for patients with functional and degenerative MR are similar to surgery, but worse overall for functional compared to degenerative aetiologies (Figure 3) due to the inherently higher risk profile of functional MR patients.
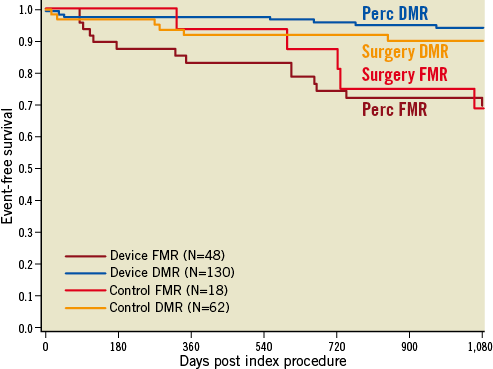
Figure 3. Kaplan-Meier freedom from mortality comparing functional and degenerative MR patients from the EVEREST II randomised trial. Compared to surgical mitral valve repair or replacement, neither the degenerative nor functional patients’ event-free survival differed when treated with MitraClip or surgery39. Perc: percutaneous; DMR: degenerative MR; FMR: functional MR
A high-risk registry demonstrated improved one-year mortality in these high-risk, predominantly functional MR patients in comparison to a non-randomised concurrent control group5. Possibly more importantly, a reduced rate of repeat hospitalisation in the year following MitraClip placement was also demonstrated. This clearly is consistent with both a quality-of-life and an economic benefit for MitraClip therapy in these patients.
Those who have failed cardiac resynchronisation therapy form a special subgroup of patients. While responders to cardiac resynchronisation therapy have a good response in terms of MR reduction, non-responders have a dismal prognosis. One series of patients, cardiac resynchronisation therapy non-responders with severe MR who underwent MitraClip therapy, showed improvements in ejection fraction and favourable left ventricular remodelling after MitraClip therapy6.
Despite the fact that the MitraClip produces a tissue bridge between the leaflets7,8, surgical repair remains possible after MitraClip therapy9,10. It is clear that repair is more difficult after a mitral clip device has been placed. Successful repair has been accomplished as long as five years after clip implantation. It is critical to understand how to unlock the device so that it can be removed during the course of repair surgery. Infrequently, prior MitraClip placement will result in replacement of a valve that might have been repairable initially. Generally, valves that are replaced after MitraClip device implantation have morphologic features, such as leaflet or annular calcification that are predisposed to replacement when mitral valve operation is the initial intended therapy.
Recognition that the best results from MitraClip are in higher-risk patients with functional MR has therefore resulted in application of this therapy (Figure 4). Numerous registries have been reported, with safety and efficacy that is similar to that reported in the initial EVEREST high-risk registry11-20. The EVEREST II high-risk registry demonstrated similar safety to the randomised EVEREST II trial as well as efficacy assessed by a decreased heart failure rehospitalisation rate and decreased mortality compared to a non-randomised concurrent control group. To evaluate the safety and effectiveness of the MitraClip system for the treatment of moderate-to-severe or severe functional MR in symptomatic subjects who are extremely high risk for mitral valve surgery, a second randomised controlled trial is being undertaken, namely the Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for High Surgical Risk Patients (COAPT) Trial: a clinical evaluation of the safety and effectiveness of the MitraClip system for the treatment of functional MR in symptomatic subjects who are extremely high risk for mitral valve surgery. It will be a prospective, randomised, parallel-controlled, multicentre clinical evaluation with subjects randomised in a 1:1 ratio to the MitraClip device or to a no MitraClip device control group who will receive medical therapy. Randomisation will be stratified by study site and cardiomyopathy aetiology, ischaemic or non-ischaemic. The primary safety endpoint is a composite of all-cause death, stroke, worsening kidney dysfunction, permanent left ventricular assist device implant, or heart transplant at 12 months. For inclusion, patients must be treated with optimal medical therapy including cardiac resynchronisation therapy, have a heart failure hospitalisation within the previous year or an elevated BNP, and have mitral valve leaflet morphology suitable for the MitraClip device. The primary effectiveness endpoint is recurrent heart failure hospitalisations. A similar European trial, RESHAPE, will also be undertaken.
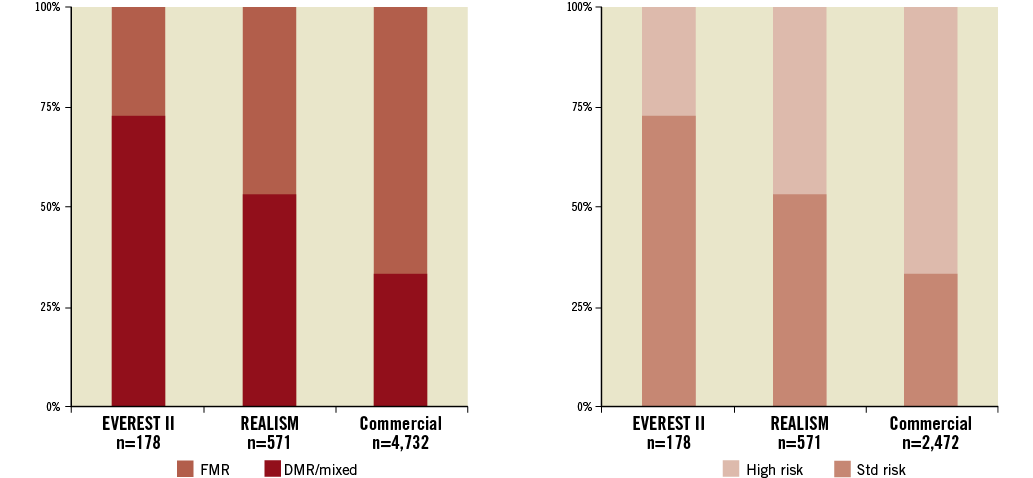
Figure 4. (Left) The randomised EVEREST II trial primarily treated patients with degenerative MR (DMR), who were standard operative risk and representative of patients who have traditionally been treated with surgery. The proportion of patients with functional MR (FMR) has increased steadily as trial experience has been gained. (Right) High-risk profile of patients selected for MitraClip has steadily increased. In “real-world” practice currently the vast majority of patients are high risk for surgery. The patients analysed in the EVEREST high-risk registry represented a high-risk subset taken from the REALISM registry35. DMR: degenerative MR; FMR: functional MR
The EVEREST randomised trial and high-risk registry data were recently reviewed by the FDA circulatory devices panel21. The comments prepared by FDA prior to the panel meeting concluded that the EVEREST II randomised trial did not demonstrate an appropriate benefit-risk profile when compared to standard mitral valve surgery in a selected mitral valve patient population. They stated that the EVEREST II high-risk registry data are not easily interpretable and represent a continued access protocol cohort that was not intended to be used as a pivotal data set, and that pooling of the registry data sets in a post hoc manner has major design limitations. FDA believes these analyses do not constitute valid scientific evidence of safety and effectiveness for the proposed indication for use in an inoperable MR population. Despite the FDA comments, the advisory panel voted narrowly in favour of a reasonable benefit-risk profile for the proposed indication22, for the percutaneous reduction of significant symptomatic MR ≥3+ in patients who have been determined by a cardiac surgeon to be too high risk for open mitral valve surgery and in whom existing comorbidities would not preclude the expected benefit from correction of the MR. At the time of writing, FDA has yet to make a final statement regarding approval or indications for MitraClip use in the United States.
Annuloplasty approaches
The second largest human experience with percutaneous mitral repair utilises the CARILLON Mitral Contour System (Cardiac Dimensions, Inc.) (Figure 5)23,24. This is a coronary sinus annuloplasty device. The procedure involves jugular venous access and cannulation of the coronary sinus to deliver the wire-form device. This shortens the circumference of the coronary sinus, which encircles the mitral annulus. Tethering of the device results in a significant diminution of the circumference of the coronary sinus.
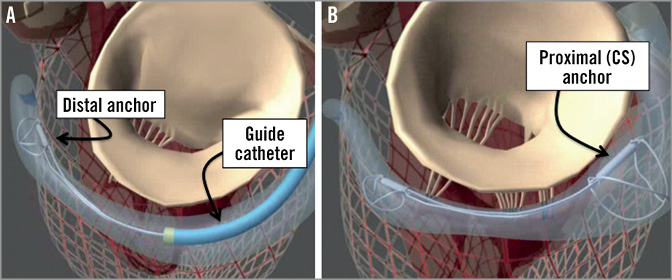
Figure 5. Coronary sinus (CS) annuloplasty with the CARILLON device (Cardiac Dimensions, Inc.). A guide catheter is engaged in the CS and the device is anchored in the distal CS or great cardiac vein. The guide and device are pulled back together to cinch the annulus, and the proximal anchor is then released in the CS ostium35.
The device has recently been reported in the TITAN trial. Fifty-three patients were included in the trial. Thirty-six were implanted with the device, and 17 could not be implanted due either to difficulty cannulating the coronary sinus, or in some cases to compression of the circumflex coronary artery, which may cross under the coronary sinus in a significant number of patients. One-year results of this trial showed a significant decrease in indices of MR severity, left ventricular chamber reverse remodelling, and clinical measures of functional improvement.
Compared to baseline, these measures were all favourably progressive throughout the course of the first year after therapy. The non-implanted comparator patients, of course, had no improvements in any of these parameters, and the implanted patients had significant improvements in comparison to the non-implanted patients. This device has received CE mark approval in Europe, and is commercially available. Further study in a randomised comparison with medical therapy in a heart failure population will be necessary to define the role of this therapy further.
The coronary sinus approach is indirect. The coronary sinus is often as much as 1 cm above the mitral annulus25. In addition, in many cases the coronary sinus crosses over the circumflex coronary artery or one of its branches, leading to significant concern regarding coronary artery compression. In fact, for some of the devices previously utilised in the coronary sinus, in rare cases myocardial infarction resulted from coronary artery compression26. It is thus attractive to consider approaches that would result in direct annuloplasty. Several devices accomplish this. Currently three devices are under development for direct annuloplasty. The Mitralign device (Mitralign, Inc., Tewksbury, MA, USA) uses a retrograde transventricular approach to place a guide catheter under the mitral annulus behind the posterior mitral leaflet (Figure 6)27. Radiofrequency wires are used to traverse the true annulus. Pledgets are placed over the wires. Two pairs of pledgets are used, and each pair can be pulled together to cinch or tether the mitral annulus to shorten the mitral circumference. The degree to which annular shortening is accomplished depends on the ability to place two pairs of pledgets. Early experience suggests that some patients may have an adequate reduction and MR from placement of a single pair. This device is enrolling patients in a European CE mark approval trial. There is considerably less human application of another cinching device, the Accucinch device (Guided Delivery Systems, Inc., Santa Clara, CA, USA) (Figure 7). The Accucinch is similarly delivered via a retrograde transventricular approach. Multiple nitinol anchors are placed in the basilar left ventricular myocardium beneath the posterior mitral leaflet. A tether or cord is anchored by these nitinol wires, and is then pulled to cinch and shorten the mitral annulus. This device has the advantage of remodelling both the basilar left ventricle and the mitral annulus, distinguishing it from purely annular devices. A transatrial septal annuloplasty ring has been developed by Valtech (Figure 8)28. A transseptal guide catheter is used to deliver a partial ring to the atrial side of the annulus. The device is anchored using multiple screws. This device is still at the first-in-human stage of development.
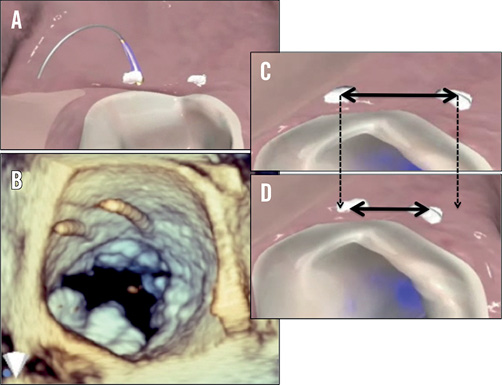
Figure 6. Direct annuloplasty with the Mitralign system. A pair of pledgets is placed in the mitral annulus near one commissure and drawn together to shorten the mitral annular circumference. A second pair of pledgets is placed adjacent to the other commissure. A and B show the wires used for passage of the pledgets. C and D show shortening of the tissue between the pledgets35.
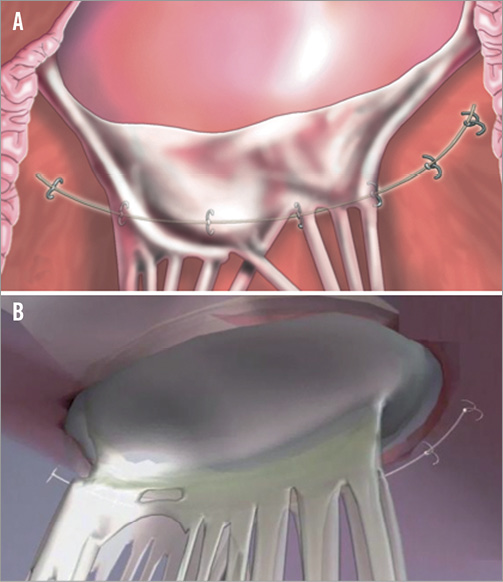
Figure 7. The Accucinch (Guided Delivery Systems, Inc.) places a delivery catheter under the posterior leaflet and then delivers anchors into the annulus. The anchors are drawn together with a cord or tether. There is some remodelling of the base of the LV as well as the annulus35.
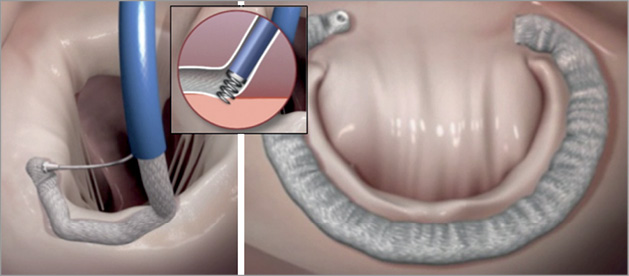
Figure 8. The Cardioband (Valtech Cardio, Or Yehuda, Israel) is a direct annuloplasty device that is placed from a transseptal approach and anchored directly into the mitral annulus on the atrial side35. The left panel shows the ring being extruded from the delivery catheter. The inset shows the anchoring screw mechanism. The right panel shows the completed implant.
Devices that have gone by the wayside
Many lessons can be learned from the percutaneous mitral repair devices that have already gone by the wayside. Several failure modes have stopped development of a variety of percutaneous mitral repair devices. Percutaneous transcoronary sinus mitral annuloplasty with the Viacor device (Viacor, Inc., Wilmington, MA, USA) represents one of these failed developments29. The Viacor device consisted of a flexible catheter delivered into the coronary sinus, into which relatively rigid nitinol rods were passed to compress the coronary sinus in the septal-lateral dimension. The rods were prone to fracture, and in one case a rod eroded through the plastic delivery catheter and lacerated a lung30. This highlights the torsional motion and device stresses that are found in the coronary sinus, which was not an anticipated problem. Fracture of nitinol was also part of the end of development of the Edwards MONARC device (Edwards Lifesciences, Irvine, CA, USA)31. This was a coronary sinus annuloplasty device comprising two self-expanding stents connected by a spring-like bridge element32. This device was prone to fracture at the junction between the spring bridge connector and one of the stents.
Leaflet repair represents another arena where failures of the past highlight challenges in the field. The Edwards Mobius leaflet repair system (Edwards Lifesciences) was designed, as the MitraClip has been, to reproduce the surgical Alfieri edge-to-edge surgical repair33. While the surgical repair uses pledgeted sutures to approximate the free edges of the mitral leaflets, the Mobius device required a catheter placement of suture. Accurate placement of the suture proved to be a great challenge, as did the consistent capture of large enough amounts of tissue to make the suture stable. Annular remodelling with radiofrequency has similarly been explored and not developed.
One of the most interesting developments that did not reach fruition is illustrated by the Coapsys system (Myocor®, Inc., Maple Grove, MN, USA)34. This concept involved placement of epicardial pads on the anterior and posterior left ventricular surfaces, connected by a transcavity cable. The cable tensioned the pads and pressed both the mitral annulus and the left ventricle. Thus, this was the first device to result in both mitral and chamber remodelling. In a randomised surgical trial, comparing the Coapsys device with conventional annuloplasty, the Coapsys device resulted in a better one-year survival than conventional surgery for ischaemic MR. Chamber remodelling was sustained by this device. Unfortunately, the financial crisis in 2008 led to insufficient funding for the company and further development halted. Importantly, two percutaneous procedures utilising the Myocor device and concept had been accomplished at that point. The devices that have achieved significant clinical use are now leaflet repair with the MitraClip (Abbott Vascular) and the CARILLON coronary sinus annuloplasty device (Cardiac Dimensions Inc.).
Devices under development
A variety of new devices are under development35. One novel approach, the mitral spacer, uses a spacing balloon anchored to the left ventricular apex on the endocardial surface. The balloon “floats” in the left ventricle, and spans the line of mitral closure, occupying the space of the regurgitant orifice. The concept has not yet been tested in patients. Chordal replacement has been used in a surgical beating heart approach. Transapical placement of Gore-Tex neochordae (W.L. Gore & Assoc, Inc., Flagstaff, AZ, USA) can be effectively performed36. Considerable development will be needed to make this a percutaneous procedure. Another novel concept for ischaemic MR, the Mardil device (Mardil Medical, Inc., Plymouth, MN, USA), places a belt-like band around the left ventricle. It is adjusted by inflation with fluid to decrease the annulus size, minimising functional MR. The device addresses the cause of functional MR by supporting the annulus and subannular region of the ventricle and papillary muscles. Several patients have been treated with a surgical implant version of this device.
Percutaneous valve replacement
Percutaneous mitral valve replacement is also being developed37. The rapid introduction of transcatheter aortic valve replacement has for many people created the assumption that mitral valve replacement could develop just as rapidly. This is clearly not the case. Several challenges are faced by percutaneous mitral replacement. In contrast to the aortic valve, there is no heavy calcification to facilitate anchoring of the valve frame. Accordingly, anchoring mechanisms are one of the important challenges to this technology. In addition, the mitral orifice has a large, not round shape. Valvular leaks at the corners of the valve are an important challenge. Only a few patients have been treated with percutaneous mitral replacement to date and, while the technology will clearly emerge, it may take significantly longer than was seen with transcatheter aortic valve replacement.
Conflict of interest statement
T. Feldman is a consultant for and receives research support from Abbott Vascular, BSC, and Edwards. A. Young has no conflicts of interest to declare.

