Abstract
Aims: The long-term results of bare stenting in complex procedures are troubled by a high incidence of restenosis. The use of drug-eluting stents lead to excellent long-term angiographic results, but require, long-term antithrombotic treatment to prevent stent thrombosis. We evaluated the efficacy of high-dose atorvastatin as an adjuvant in limiting clinical and angiographic restenosis after bare stenting procedures which carry an high risk of restenosis.
Methods and results: Between November 2003 and February 2004, 91 consecutive patients (with 152 target lesions) underwent successful interventional procedures with bare stents (158 stents; 1.7 stent/patient). The patients were included in the study in the presence of at least one of the following criteria: diabetes mellitus (14.6%); acute myocardial infarction (8.8%); lesion length >15 mm (27.5%); lesion in vessels with a reference diameter of <2.5 mm (24.2%); >2 treated lesions (58.2%); side-branch lesions (3.3%). All patients were started on atorvastatin (80 mg/day) on the day of the procedure, and continued receiving it for at least nine months.
Six of the 91 patients (6.5%) experienced a clinical restenosis and underwent target lesion revascularisation; a further two underwent re-PTCA due to progressive atherosclerosis in untreated sites. Seventy-one patients (78.0%; 125 stents) were followed up with angiography after a mean 8.5 months (7-10 months): binary angiographic restenosis was observed in 18/71 patients (25.3%) (in-stent restenosis 21.1%; in-segment restenosis 4.2%), and 17/125 stents (13.6%).
We matched 122 lesions (125 stents) from the present population with a reference group of 600 consecutive lesions treated at the Ospedale Maggiore della Carità in Novara during the same period of time, but without high-dose statin treatment: 88 lesions (94 stents) were successfully matched on the basis of the clinical and angiographic variables of diabetes, acute myocardial infarction, treated vessel, reference diameter and lesion length. The incidence of late loss was significantly lower in the atorvastatin group (p=0.027).
Conclusions: The RESTART prospective study showed that the adjunct of atorvastatin 80 mg leads to excellent clinical and angiographic outcomes. These results were confirmed in a matched lesion comparison.
Introduction
Stent deployment reduces late restenosis in selected patients in comparison with balloon angioplasty; however stenting procedures are still limited by a restenosis rate that exceeds 30% in unfavourable lesions1. Drug-eluting stents (DES) suppress the process of in-stent restenosis (which consists of smooth muscle cell proliferation and extracellular matrix formation within the stent struts), and can therefore be extensively used in complex procedures2-5. However, the long-term need for antiplatelet therapy to prevent late DES thromboses and the marked increase in financial costs are two good reasons for considering alternative solutions based on oral drug administration. It has been found that high-dose statin therapy inhibits smooth muscle cell proliferation in animal models and preliminary clinical studies6-7. The aim of this study was to evaluate the efficacy of atorvastatin 80 mg in reducing the incidence of clinical and angiographic restenosis after bare stenting procedures carrying a high risk of restenosis.
Methods
Patient population
Between November 2003 and February 2004, 164 coronary angioplasties were performed at our centre, of those 91 consecutive complex procedures entered our prospective study and were treated with bare stents. The patients received atorvastatin 80 mg in addition to standard therapy from the day of stenting and until scheduled follow-up coronary angiography. The patients were included in the study in the presence of at least one of the following clinical, angiographic and procedural criteria: diabetes mellitus, acute myocardial infarction, lesion length > 15 mm, small-vessel lesions (reference diameter < 2.5 mm), treatment of at least two lesions, and side-branch lesions. The exclusion criteria were: the use of a DES, concomitant diseases such as renal insufficiency (serum creatinine >1.5 times the upper normal limit), liver dysfunction (SGOT and/or SGPT values more than twice the upper normal limit and/or serum total bilirubin >1.5 times the upper normal limit), biliary obstruction, pancreatitis, and untreated hypo- or hyperthyroidism; any condition that would require the use of a concomitant medication known to affect lipid profile during the study; and a history of drug or alcohol abuse within the previous five years.
The patients undergoing angiographic follow-up were matched with a reference group of 600 consecutive patients who underwent bare stenting for complex procedures and were not treated with a high-dose statin (>40 mg). The reference group consisted of patients who underwent interventional procedures between June 2003 and June 2004 at Ospedale Maggiore della Carità in Novara, which scheduled follow-up angiograms in all treated patients and thus enabled the matched comparison. Matching was based on principles derived from the Thoraxcenter group (Rotterdam, The Netherlands): (1) the angiographic dimensions of matched lesions are assumed to be “identical”; (2) the observed differences between the two identical lesions must be within the range of reproducibility of the quantitative angiographic analysis system.
Matching was performed by a computerised program that iteratively scanned the two databases chronologically and selected for each lesion in the Rome database the first lesion encountered in the Novara database that satisfied the selection criteria. The matching process was aimed at comparing late loss with quantitative coronary angiography (QCA), and included the following clinical and angiographic variables: diabetes, acute myocardial infarction, treated vessel, reference diameter and lesion length8,9.
Stenting procedures and medication protocols
All patients in the registry underwent elective stenting of native coronary artery lesions, in which new generation stents with a cellular design were deployed at an inflation pressure of >10 atm. Heparin 5000 IU was administered after the insertion of the arterial sheath, and an additional bolus was given when necessary to maintain an activated clotting time of >250 sec.
Intracoronary nitroglycerin 200 µg was given before the acquisition of the baseline angiogram, and repeated before the final and follow-up angiograms. After the intervention, clopidogrel 600 mg was administered for four weeks, and chronic treatment with aspirin 100 mg/day was started. Atorvastatin 80 mg was started on the day of the intervention and continued until the follow-up assessment.
Laboratory assessment
C-reactive protein (CRP) levels were measured upon admission and 72 hours after the intervention. Total cholesterol, LDL and HDL cholesterol, triglycerides, creatinine, SGOT, SGPT, CPK and total bilirubin levels were measured by a central laboratory at baseline, and after three and nine months.
Quantitative coronary angiography
QCA analysis was performed off-line as previously described using a computer-assisted system and an automated edge detection algorithm (MEDIS, Cardiovascular Angiography Analysis System II, Pie Medical Data, Maastricht, The Netherlands) at the European Imaging Laboratory in Rome, Italy, by observers who were unaware of the treatment allocation10,11. The treated segments were analysed using two orthogonal views before and after the intervention, and at follow-up. The primary angiographic endpoint of the study was late lumen loss.
Definitions
Late lumen loss was defined as the post-intervention minimal stent lumen diameter minus the follow-up minimal lumen diameter.
Angiographic restenosis was defined as a diameter stenosis of >50% at the treated site.
Clinical restenosis was defined as the occurrence of angina and/or presence of effort induced ischaemia in patients with angiographic restenosis.
Non Q-wave myocardial infarction was diagnosed by a rise in the creatine kinase-MB level to more than twice the normal limit without occurrence of Q-waves at the ECG.
Statistical analysis
The continuous variables are expressed as mean values±SD, and were analysed using the two-tailed Student’s t test. A Chi Square test was used to detect any differences between categorical variables. A p value of < 0.05 was considered statistically significant. An analysis with Student’s t test or a Chi Square test, when appropriate, was performed to identify variables which correlated with late-loss and could eventually enter in a multivariate model of analysis.
The following clinical and procedural variables were tested: diabetes, unstable and stable angina, smoking habit, use of atorvastatin 80 mg, lesion length, lesion reference diameter, lesion location in the proximal left anterior descending (LAD) coronary artery.
Results
The RESTART study
PATIENT AND PROCEDURAL CHARACTERISTICS
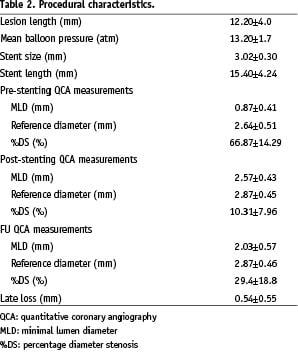
The clinical characteristics of the 91 study patients and the procedural variables are shown in Tables 1 and 2.
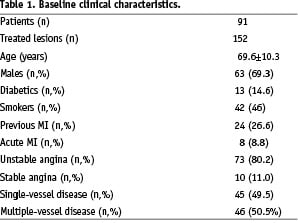
Thirteen patients (14.6%) were diabetic, three of whom (3.2%) had insulin- dependent diabetes. The vast majority of the patients were admitted because of acute coronary syndromes; only 11.0% had stable angina.
A total of 152 target lesions were treated using 158 bare stents. The intervention involved at least two lesions in 58.2% of the patients, and two and three vessels were treated respectively in 22.0% and 3.3% of the patients. The lesions were located in the LAD in 48.0% of the patients, the left circumflex (LCX) in 23.8%, and the right coronary artery (RCA) in the remaining 28.2%; 4.6% of the lesions were type A, 87.5% type B, and 7.9% type C. The interventions involved small vessels (< 2.5 mm) and long lesions (> 15 mm) in 24.2% and 27.5% of patients, respectively.
CLINICAL OUTCOME
There were no in-hospital “Q-wave AMIs”, however a “non-Q-wave AMI” occurred in 3 patients (3.3%). After discharge, one patient experienced a subacute stent thrombosis due to the interruption of clopidogrel treatment, which caused a Q-wave MI, but there were no non-Q-wave MIs during the follow-up. Six of the 91 patients (6.5%) experienced a clinical restenosis and underwent target lesion revascularisation; a further two patients underwent re-PTCA due to progressive atherosclerosis in untreated sites. The follow-up angiographic examination was performed after 8.5±2.2 months (7-10 months) in 71 patients (78.0%) with 125 stents. Binary angiographic restenosis was observed in 18/71 patients (25.3%). In-stent restenosis occurred in 15 cases (21.1%), but in-segment restenosis was less frequent (3 cases, 4.2%). Angiographic restenosis was found in 17/125 stents (13.6%), in a large proportion (64.7%) of which late neointima proliferation caused moderate narrowing of <60% (Figure 1).
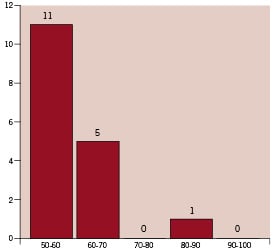
Figure 1. This refers to the 17 stents with late restenosis. The x axis shows the stents grouped on the basis of the QCA percentage narrowing measured at follow-up.
CRP measured after 72 hours was above the reference value in 29.6% of the patients who underwent follow-up angiography. The sensitivity and specificity of CRP for stent restenosis were respectively 0.75 and 0.84 (Table 3).

The matching comparison
Six hundred and fifty consecutive lesions in the reference patient group of Novara were screened. Of the 122 lesions (125 new generation stents with cellular design) in the RESTART study with an angiographic follow-up, 88 (94 stents) (Group 1) were successfully matched with 88 (95 stents) in the reference group employing same stent design (Group 2). Thirty group 2 patients (34.1%) were on statin therapy at the time of coronary intervention and during follow-up (mean duration 7.5±2.6 months). Apart from the higher prevalence of unstable angina in group 1, there were no differences between the two groups in terms of the clinical and angiographic data or the implantation technique (Table 4).
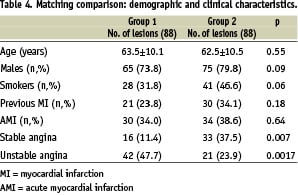
The variables used to match the two groups and their prevalence were: diabetes mellitus (14.3%), acute myocardial infarction (13.2%), lesion >15 mm (25.0%), lesion in vessels with a reference diameter of <2.5 mm (30.8%), treatment of at least two lesions (58.2%), and side-branch lesion (3.2%). The treated artery was the LAD in 45.4% of cases (11.4% of these located in the proximal segment), the LCX in 20.4%, and the RCA in 34.1%.
Follow-up QCA revealed a lower incidence of stents with restenosis in group 1 (13.2% vs 24.2%; p=0.08), which had also significantly lower late loss (0.56±0.55 mm vs 0.79±0.64 mm; p=0.025) (Table 5).
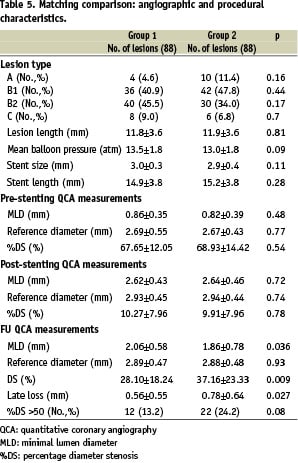
At the analyses performed to address the impact exerted by clinical and angiographic variables on late loss, use of atorvastatin 80 mg was found to be the only variable related to late restenosis (p=0.022).
Tolerability of atorvastatin and cholesterol variations in the RESTART study
Baseline total, LDL and HDL cholesterol, and triglyceride values were respectively 179.7±43.7 mg/dL, 103.5±37.2 mg/dL, 40.4±16.1 mg/dL, and 113.2±46.4 mg/dL.
The mean follow-up total and LDL cholesterol levels were significantly lower (147.7±44.4 mg/dL; p=0.001; and 75.0±24.9 mg/dL; p=0.002). The median HDL and triglyceride levels assessed during follow-up remained unchanged (41.4±10.1 mg/dL; p=0.36; and 108.9±42.1 mg/dL; p=0.21).
Atorvastatin treatment was stopped in two patients: in one, after four months because of SGOT and SPOT elevations more than twice the reference limits; in the second, after three months because of leg muscle pain without a concomitant increase in CPK levels.
In the remaining 89 patients, atorvastatin 80 mg was well tolerated and did not lead to any increase in SGOT, SGPT or CK values.
Discussion
The main finding of the present study is that atorvastatin 80 mg helps achieve excellent clinical and angiographic outcomes in patients undergoing bare stenting procedures in unfavourable clinical and anatomical situations leading to high incidence of late restenosis. The result was confirmed by a matched lesion comparison.
Rationale for statins use to inhibit neointima formation
The rationale for using statins therapy to limit restenosis is based on the potential inhibition of smooth muscle cell proliferation6,7, and the prevention of the post-intervention increase in serum CRP levels12,13.
Indolfi et al7 showed that high-dose 3-hydroxy-3-methylglutaryl coenzyme A inhibitor therapy prevents vascular smooth muscle cell formation after vascular injury. The authors assessed the impact of simvastatin 40 mg/kg/day on the in vivo proliferation of smooth muscle vessel’s cells by means of balloon injury and stent application in the common carotid arteries of rats, and found that it led to a significant (66%) reduction in late in-stent neointima formation.
Analysis of the CARE study12 revealed that randomisation to pravastatin 40 mg/day led to a 7% decrease in serum CRP levels. As it has been shown that cardiac events (used as surrogates for restenosis) are more frequent in patients with high CRP levels 48 and 72 hours after stenting, a statin-induced reduction in serum CRP levels may have a favourable long-term effect.
Clinical studies of statins therapy after balloon angioplasty and stenting
There is a burden of evidence to suggest that statin therapy exert a beneficial effect after percutaneous coronary intervention14,15. Chan et al14 studied 1,552 consecutive patients, treated with angioplasty and prospectively followed for 1 year. Statins pretreatment was an independent predictor for 1-year survival within the highest hsCRP quartile.
Studies of the impact of lipid-lowering drugs on the reduction of restenosis after balloon angioplasty led to conflicting results16-18. Statins therapy did not consistently reduce restenosis after balloon angioplasty. The mechanism of restenosis in this setting is late vessel recoil (which accounts for more than 60% of late lumen loss and is not affected by statin therapy). These studies were not designed to assess the effect of statins on late neointima in-growth, which is the main mechanism involved in post-stenting late restenosis because the scaffolding properties of the endoprotheses prevent late vessel recoil19.
The first demonstration of a statin-induced benefit on late in-stent restenosis was provided by Walter et al20, who retrospectively analysed the angiographic results of 525 consecutive stented patients and found a lower restenosis rate in those treated with statins (25% vs 38%). However, a major drawback of the study was that the patients were treated with different statin regimens and, as 19% were already receiving statin therapy, it was not clear if statin pretreatment was necessary to improve the post-stenting restenosis rate.
Our group has more recently tested the efficacy of pravastatin 40 mg in controlling the restenosis rate of stented lesions in a comparative, non-randomised, intravascular ultrasound (IVUS) study21. Eighteen consecutive stents treated with pravastatin 40 mg, and 25 consecutive stents untreated with lipid-lowering drugs, underwent IVUS assessment after 12±3 months, which showed a significant reduction in the amount of neointima in the patients receiving pravastatin (21% vs 29%; p < 0.03).
The long-term results of the RESTART study
To the best of our knowledge, this is the first prospective study of the effect of high-dose statins on in-stent restenosis in a subset of patients with complex lesions. Interestingly, our results mirror those of the TAXUS IV trial5, which addressed the impact of DES in a broad subset of lesions that also included long stenoses and small vessels. Repeat target lesion revascularisation was necessary in 6.5% of our patients, which compares well with the 3.3% incidence reported in the bare arm of TAXUS IV.
We found a discrepancy between the prevalence of clinical and angiographic restenosis as 25.3% of the patients showed a late narrowing of >50% in at least one deployed stent. It is worth noting that the neointima in-growth led to a <60% luminal reduction in the vast majority of the stents with late restenosis. When late restenosis is between 50% and 60%, effort-induced ischaemia may not occur because stent scaffolding prevents variations in vessel wall tone. Alternatively, statins may have an effect on microvascular circulation because of their ability to enhance the ischaemic threshold.
The finding that 25.3% of the patients showed late restenosis should not be considered a cause of concern because it reflects the complexity of our population (58% of the patients were treated with more than one stent). In fact only 13.6% of the 158 implanted stents showed restenosis at follow-up angiography, and the group treated with atorvastatin 80 mg experienced a 45.4% reduction in late restenosis and a 29.1% reduction in late loss.
A finding of high CRP values despite high-dose atorvastatin accurately identifies in-stent restenosis. In this unfavourable setting, the use of a treatment protocol based on the concomitant administration of oral corticosteroids and statins may further improve the restenosis rate11.
Clinical impact
Drug-eluting stents halt in-stent neointima proliferation and dramatically reduce the incidence of restenosis, but their extensive use requires caution based on financial concerns and recent pathological findings of an association between delayed endothelisation and a higher rate of stent thrombosis as compared to bare stents22. The use of high-dose statins during interventional procedures using bare stents rather than DES may therefore represent an alternative solution to costly procedures possibly carrying a higher risk of late endoprostheses thrombosis.
The inhibition of neo-intima proliferation suggested in our study is an adjunctive effect, as the marked lipid-lowering and anti-inflammatory action of atorvastatin 80 mg improved clinical outcome and helped control the progression of atherosclerosis. Our findings therefore further encourage the use of aggressive high-dose statin treatment in patients referred for complex stenting procedures.
Limits
The current study uses a validated method of matching comparison8,9 between stenting procedures performed at two different centres instead of the more traditional double blind placebo control single centre study. In theory these procedures might have been performed at two centres adopting different technical solutions, based on their peculiar experience. However the adopted matching process, based on anatomical, clinical and procedural characteristics, likely enabled an accurate comparison between two similar groups of patients.
A number of clinical and angiographic parameters well known to affect the restenosis rate after stenting procedures were found to be inconsequential in this study. The explanation seemingly rests in the small number of patients with these characteristics in both groups of patients. Some parameters, in fact, which were more represented (length of the lesions, diabetes) showed a trend toward affecting the restenosis rate consistent with the data in the literature. However, the study was primarily tailored to study the effect of high dose atorvastatin on restenosis rate.

