Abstract
France was chosen to be one of the six first pilot countries of the “Stent for Life” European initiative. First, a prospective registry was set up in five representative French regions, including all admissions within the first 48 hours of ST-elevated acute cardiac syndrome between 1st and 30th November 2010. The second step was to improve results. The main objective was to encourage members of the public experiencing chest pain to call immediately the SAMU’s direct line (phone number “15”). Another action was to organise medical meetings in order to improve the management of these patients. Letters were also sent to general physicians to alert them to the issue and to the Stent for Life project. The third step consisted of creating a new registry, in November 2011, to assess the impact of the above actions on an area basis. It has resulted in streamlining the networks and bringing the rate of non-reperfusion down below the 10% threshold. Much remains to be done to improve public awareness of life-saving actions.
Introduction
In Europe, the quality and efficacy of ST-elevation myocardial infarction (STEMI) management varies widely from country to country, as underlined by the work of Widimsky et al1, France being among the countries with room for improvement. The 2005 FAST MI registry2 concluded that management of acute MI in France was not optimal: almost 40% of acute MI patients did not undergo primary PCI or thrombolysis at all, even during the first hours. France was therefore chosen to be one of the six pilot countries in the European Association of Percutaneous Cardiovascular Interventions (EAPCI)’s Stent for Life project, intended to improve and harmonise management. In France, Stent for Life was launched jointly by the French Society of Cardiology (SFC), the French Federation of Cardiology (FCF), the French Society for Emergency Medicine (SFMU) and the Emergency Medical Assistance Service (SAMU Urgence de France).
Methods
POPULATION
Five representative administrative geographical areas (départements) of France were selected according to surface area, population density, hospital accessibility, number of non-stop percutaneous coronary intervention (PCI) centres, and number of centres with intensive care units but no PCI facility. The selected départements were Nord, Essonne, Côte d’Or, Haute Savoie and Haute Garonne (Table 1, Figure 1).
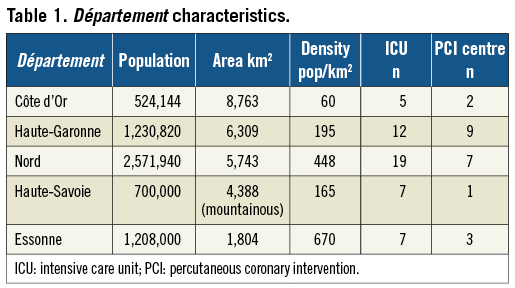
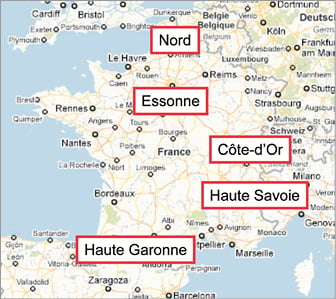
Figure 1. Selected “départements” (administrative geographic division of France).
First, a prospective registry was set up in the five areas, including all admissions within the first 48 hours of ST-elevated acute cardiac syndrome (ACS) between 1st and 30th November 2010.
The second step was to improve results. A national press conference was held in March 2011. Regional press conferences (medical and public media) were held in the five areas. The main objective was to encourage members of the public experiencing chest pain to call the SAMU’s direct line (phone number “15”) immediately. Another action was to create a cell-phone application explaining what to do in case of chest pain. Medical meetings were held in each of the areas to organise and improve the management of these patients. Letters were also sent to general physicians to alert them to the issue and to the Stent for Life project.
The third step consisted of creating a new registry, in November 2011, to assess the impact of the above actions on an area basis.
DATA COLLECTION
All data were recorded on computerised case-record forms by dedicated research technicians. Cardiovascular history, risk factors, time to first call, time to first medical contact (FMC), time to reperfusion, type of reperfusion (thrombolytic therapy [TL] or primary percutaneous coronary intervention [PPCI]), and “15” calls to the SAMU mobile intensive care unit system or other were recorded for each patient.
Time to first call was defined as the time when the patient or his or her relatives first sought medical attention. Time to reperfusion was defined as time to first intravenous thrombolytic injection, or arterial puncture in patients treated by PPCI.
STATISTICAL ANALYSIS
Continuous variables were described by mean±SD, and categorical variables by absolute and relative frequency distributions. Group comparisons used unpaired t-tests for continuous variables and c² tests for discrete variables.
Results
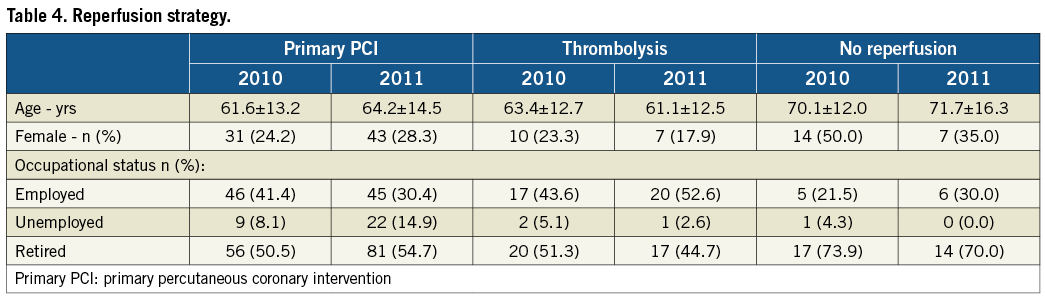
Baseline population characteristics in the two registries, summarised in Table 2, were comparable. There was no change between the two registry time-points in terms of “15” call rate or time to first call (Table 3). There was, however, a reduction in the interval between FMC and admission, from 178 minutes to 112 minutes. The number of patients not receiving reperfusion was 14.1% in the first registry, falling below the 10% threshold in the second. The percentage of women in the non-revascularised subgroup fell from 50% to 35% (Table 4).
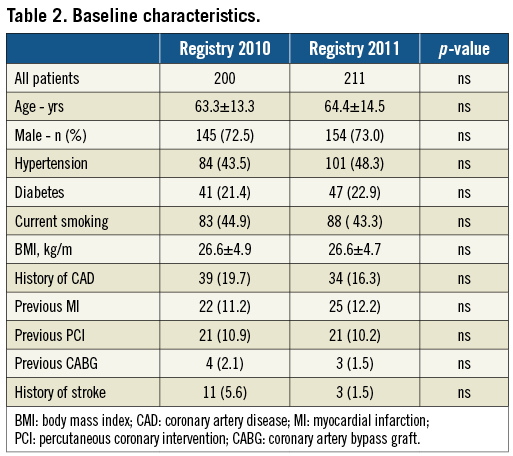
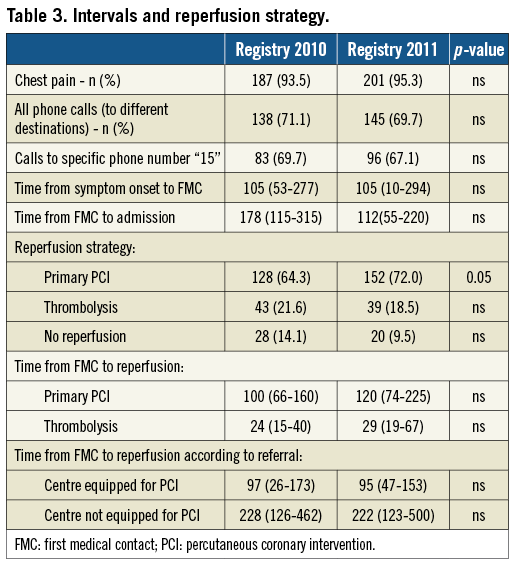
In both registries, time to treatment was much shorter in case of direct referral to a centre performing PCI compared to centres not equipped for PCI, with a mean interval of 97 minutes and a maximum of 228 minutes.
Discussion
The Stent for Life survey reports data for STEMI patients in five representative geographical areas in France in 2010 and evolution subsequent to implementation of a campaign of press conferences and meetings to improve the medical circuits concerned and to raise public awareness of the need to use the “15” telephone service.
The Stent for Life registry data for November 2010 already showed clear improvement in the management of ST-elevation ACS in France compared to the 2005 data of the FAST MI registry2: the PCI rate rose from 33% to 64.3% and the non-reperfusion rate fell from 38% to 14.1%. These findings were confirmed by the second FAST MI registry for the period October to December 2010, which included 4,170 patients in 213 centres throughout France, 60 of which were not equipped for PCI. This further confirmed that the five areas used in the present study are representative of France as a whole.
Stent for Life’s action on the medical networks can also be seen to have resulted in a clear improvement, with the non-reperfusion rate falling below the 10% threshold, the decrease of time between FMC and admission, and the rate of PCI increasing significantly. Information to medical teams likewise helped induce a fall in the percentage of women not receiving reperfusion, from 50% to 35%. The “15” emergency call service provides clear benefit, avoiding referral to centres not equipped for PCI and thus very significantly reducing delay.
Stent for Life had a significantly positive impact on medical management, but not on the public’s use of the “15” emergency call service. The media campaigns were only occasional, and failed to make the public fully aware of the need to make use of the quite remarkable service that the SAMU system provides in France: it was still called in only 50% of cases. If calling “15” could become a reflex reaction, there would be considerable further improvement in the situation, with reduced delay and less iterative referral, which are known to affect mortality rates.
Conclusion
The Stent for Life campaign in France has given our teams a great opportunity to be alert to the need to improve management of ST-elevation ACS. It has resulted in streamlining the networks and bringing the rate of non-reperfusion down below the 10% threshold. Much remains to be done, however, to raise public awareness of life-saving actions, notably including phoning “15”.
Funding
All the registry steps were funded by Abbott Vascular, Daiichi-Sankyo and Lilly, Boston Scientific, Medtronic, Cordis, Biosensor, Biotronik, Philips, The Medicines Company, Hexacath, Terumo.
Conflict of interest statement
The authors have no conflicts of interest to declare.

