
Abstract
Aims: Renal sympathetic denervation (RDN) is being studied as a therapeutic option for patients with therapy-resistant hypertension. It remains unclear if the procedure affects the renal arteries in such a way that luminal narrowing might occur at the mid to longer term. The aim of the present study was to assess renal artery integrity accurately at the medium to long term using recently validated quantitative magnetic resonance angiography software in patients treated with four different RDN devices.
Methods and results: In a prospective cohort of 27 patients referred for RDN, quantitative magnetic resonance angiography (MRA) was used to assess 52 vessels at baseline, six, and 12 months post treatment with one of four different devices. No renal artery stenosis was seen at six or 12 months. The average mean lumen area was 26.6±7.3 mm2 at baseline versus 25.0±7.1 mm² and 25.0±6.1 mm² at six and 12 months, respectively, resulting in a late loss of 1.6 mm2 at six months and 1.9 mm2 at 12 months. No differences were observed in the arterial response to RDN with the four different systems used. There was no correlation between post-procedural dissections, oedema or thrombi as detected with invasive imaging, and luminal narrowing at follow-up.
Conclusions: Quantitative MRA of patients treated with RDN revealed no significant change in renal artery dimensions up to 12-month follow-up. The lack of a change in renal artery luminal dimensions was irrespective of the arterial response to the individual devices used.
Abbreviations
CTA: computed tomography angiography
GFR: glomerular filtration rate
IVUS: intravascular ultrasound
MRA: magnetic resonance angiography
OCT: optical coherence tomography
RDN: renal sympathetic denervation
Introduction
Controlling blood pressure in hypertensive patients remains a challenge for all physicians, and treatment targets are usually not achieved despite multiple antihypertensive drugs1. Percutaneous renal sympathetic denervation (RDN) is currently being studied as a potential treatment option in patients with therapy-resistant hypertension. The indication spectrum is currently even being expanded towards several new indications2-5. At present, over 53 devices are available to dismantle afferent and efferent renal nerves in the renal arterial wall. Although the body of evidence supporting the efficacy of several of both the first- and second-generation devices is growing, long-term safety data are limited6-8, mostly restricted to duplex ultrasound findings, showing a low rate of adverse events, both at short- and longer-term follow-up7,9. A limited number of computed tomography angiography (CTA) or magnetic resonance angiography (MRA) follow-up observations have revealed an absence of renal artery stenosis at six months10. However, recent high-resolution intravascular imaging findings performed directly post procedure demonstrated signs of small thrombi and/or dissections in almost 50% of the treated arteries, illustrating that binary outcome parameters of stenosis might not be sufficient11. The aim of the present study was to assess renal artery integrity accurately at the medium to long term using recently validated quantitative magnetic resonance angiography software in patients treated with four different RDN devices12,13.
Methods
PATIENT SELECTION
We present a prospective study in which a total of 29 patients with therapy-resistant hypertension, vasospastic angina and/or heart failure underwent RDN at the Erasmus Medical Center, Rotterdam, the Netherlands, between December 2012 and February 2014. RDN was performed using one of four different systems: Symplicity Flex™ (Medtronic, Minneapolis, MN, USA), Paradise® (ReCor Medical, Palo Alto, CA, USA), OneShot™ (Covidien, Campbell, CA, USA), and Vessix™ V2 (Boston Scientific, Marlborough, MA, USA). Two patients were excluded because of the implantation of a non-MRI-compatible pacemaker at three months (n=1) and one because of a missed follow-up scan (n=1). A total of 27 patients and 52 vessels were used for the final analyses at baseline and six-month follow-up (in two patients only one vessel could be analysed at baseline because of insufficient contrast density). For the 12-month analyses, 33 vessels remained (MRA image quality was insufficient at 12-month follow-up [n=14], CTA at 12-month follow-up instead of MRA [n=1] and no 12-month follow-up MRA at the time of this research [n=4]).
DENERVATION PROCEDURE
All patients were preloaded with 300 mg aspirin, if naïve, and advised to continue with aspirin for at least one month. Preprocedurally, 100 IU/kg heparin was administered to achieve an activated clotting time >250 s. A total of 200 mcg nitrates was locally administered before invasive imaging. Procedures were performed according to the device-specific instructions for use.
Additional periprocedural invasive imaging was performed in 19 out of the 52 vessels used for the final analysis in the present study. Targeted renal arteries were examined with an intravascular ultrasound (IVUS) system with automatic pullback at 0.5 mm/s, OPTICROSS™ (Boston Scientific, Marlborough, MA, USA), and optical coherence tomography (OCT) using the ILUMIEN™ or C7XR™ system and Dragonfly™ or Dragonfly™ Duo catheter (all LightLab/St. Jude Medical, St. Paul, MN, USA) as previously described11.
IVUS AND OCT IMAGE ACQUISITION
Targeted renal arteries were examined with the OPTICROSS IVUS system with automatic pullback at 0.5 mm/s pre and post RDN. Retrieved IVUS images were stored and analysed offline.
OCT was performed after RDN. Images were acquired using the ILUMIEN or C7XR system and Dragonfly or Dragonfly Duo catheter. The catheter was positioned distally to the examined segment (distal to the first major bifurcation) and automatically pulled back at 20 mm/s, acquiring images at 100 m/s, with simultaneous iso-osmolar contrast (Iodixanol 370, Visipaque™; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) administration at a flow rate of 4-6 ml/s, depending on artery size. The protocol used for detection of oedema, thrombus or dissection using both IVUS and OCT has been published previously11.
MAGNETIC RESONANCE ANGIOGRAPHY ACQUISITION
Magnetic resonance angiography was performed on a 1.5T MRI scanner (Discovery MR450; GE Medical Systems, Milwaukee, WI, USA) at baseline and six- and 12-month follow-up according to a dedicated RDN imaging protocol. Patients were positioned in supine position and an 8-channel cardiac coil was placed on the thorax and upper abdomen. As part of this MRI protocol, a 3D Vasc Fast TOF Spoiled Gradient Echo sequence was used to acquire images of the renal vasculature, after a test bolus procedure. Images were acquired during a breath hold. The following parameters were used: field of view: 46 cm*41.4 cm; matrix: 320*192, upsampled to 512*512; number of excitations: 0.75; pixel size: 0.89*0.89 mm; flip angle: 17 degrees; slice thickness: 1.6 mm; location per slab: 28 upsampled to 56 with ZIP2; repetition time: 3.2 ms; echo time: 1.2 ms; bandwidth: 83.33 Hz. The duration of the breath hold was approximately 20 seconds, depending on the locations per slab. A power injector (Medrad® Spectris Solaris®; Bayer, Pittsburgh, PA, USA) was used to inject gadobutrol (Gadovist 1.0 mmol/ml; Bayer, Mijdrecht, the Netherlands). Gadovist was injected, around 18 seconds before acquisition, with a dose of 0.1 mmol/kg into an antecubital vein followed by 15 ml of saline at a rate of 2.5 ml/s to visualise the renal arteries.
MAGNETIC RESONANCE ANGIOGRAPHY ANALYSIS
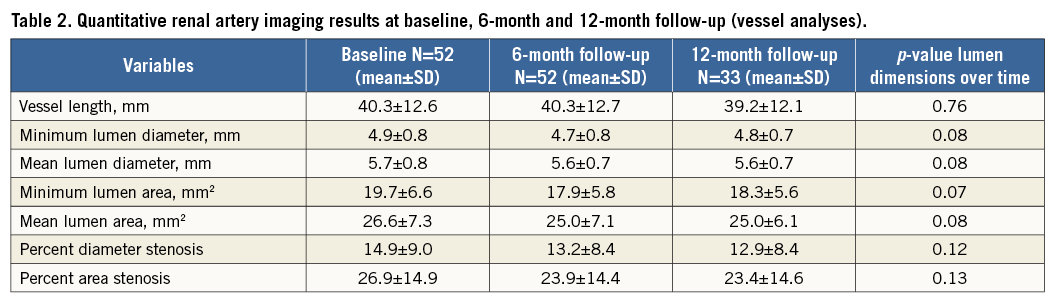
Quantitative magnetic resonance angiography was performed using dedicated imaging software, CAAS MRA, Version 1.0 (Pie Medical Imaging BV, Maastricht, the Netherlands) according to a predefined algorithm13. The automatically segmented arteries were inspected and borders were manually corrected if necessary in the stretched multiplanar rendering and perpendicular view. Vessel length (mm), minimum lumen diameter (mm), mean lumen diameter (mm), minimum lumen area (mm2), mean lumen area (mm2), percent diameter stenosis and percent area stenosis were based on semi-automatic segmentation (Figure 1). One of the investigators (L. van Zandvoort) performed all of the analyses blinded to individual patient characteristics and treatment time.
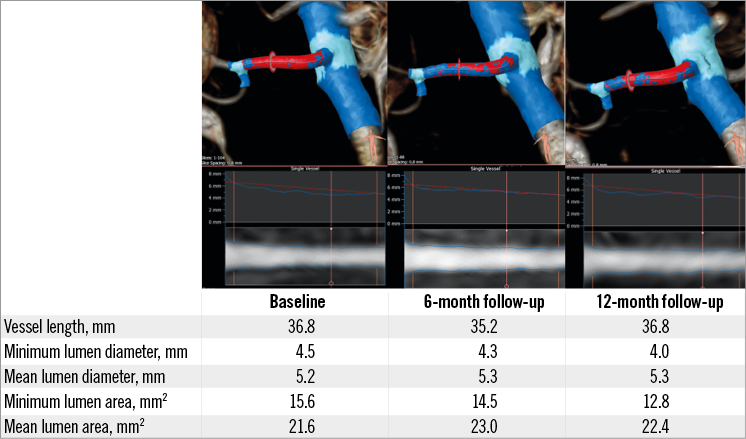
Figure 1. Case example of a renal artery analysed at baseline, six and 12 months with the use of CAAS quantitative MRA software.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA). Baseline, categorical variables are reported as either counts or percentages and compared using the chi-squared test. Continuous variables are reported as mean±SD and were compared using a linear mixed model. All continuous variables were normally distributed. Categorical variables were reported as either percentages or frequencies. A p-value <0.05 was considered statistically significant.
Between-device differences were examined using analysis of variance (ANOVA). When there was a significant difference between groups, the Bonferroni test was used as a post hoc comparison. Since our data included multiple observations per patient, crossed random effects modelling was used to account for the correlation between observations14. Luminal dimensions over time were assessed using crossed random effects with two random effects. The first random effect was the period (either six or 12 months), and the second random effect was related to whether either one or both vessels were assessed in each individual patient.
Results
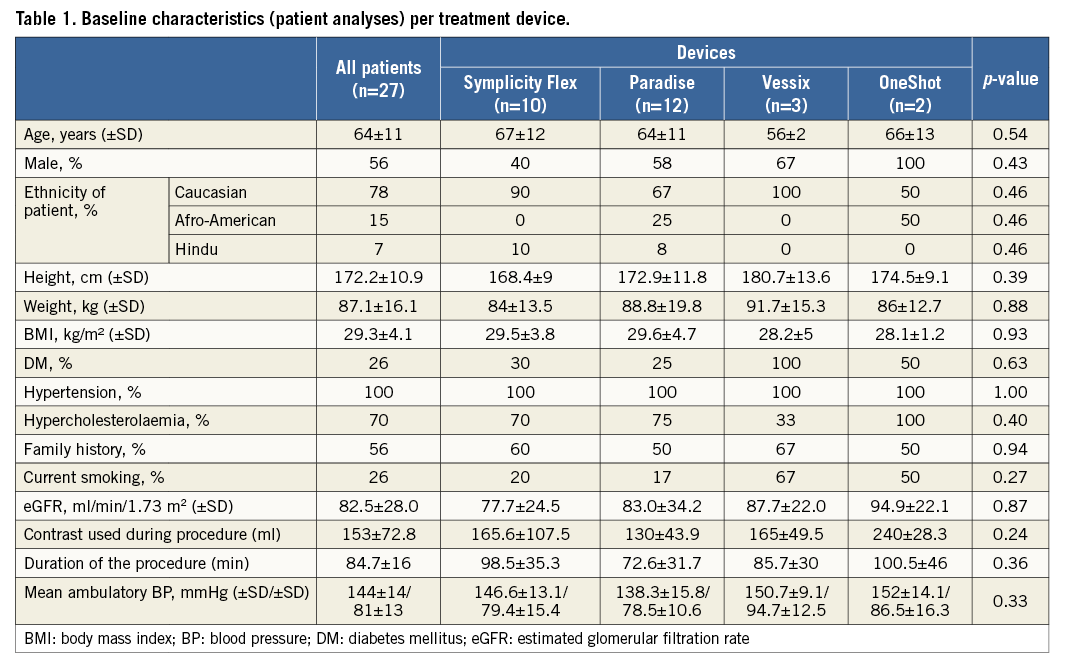
Patient demographics and baseline characteristics are depicted in Table 1. Mean age at the time of intervention was 64±11 years, and 56% of the patients were male. Diabetes was present in 26% of patients and 70% of the patients had hypercholesterolaemia. Baseline renal function was preserved in all patients with a baseline eGFR of 82.5±28.0 ml/min/1.73 m2. Mean office blood pressure (BP) was 176±21/93±17 mmHg. Mean ambulatory BP was 144±14/81±13 mmHg. There was no significant difference between any of the baseline characteristics in patients treated with the four different devices used for RDN (Table 1).
Quantitative MRA at baseline and follow-up revealed no renal artery stenosis at either six or 12 months. The average mean lumen area was 26.6±7.3 mm2 at baseline versus 25.0±7.1 mm² and 25.0±6.1 mm² at six and 12 months, respectively, resulting in a late loss of 1.6 mm2 at six months and 1.9 mm2 at 12 months (Table 2). There was no significant increase in the area or diameter stenosis over time. By using three different cut-offs for diameter and area stenosis (>25%, >50% and >70%), no trend was observed towards an increased number of patients with renal artery stenosis over time (Figure 2, Figure 3). Only five out of 52 vessels showed an absolute increase in percent diameter stenosis >25% at six months. When comparing baseline versus 12 months, no vessels were identified with a >25% increase in percent diameter stenosis. An absolute increase of >50% in percent diameter stenosis was not observed at either time point in any of the patients.
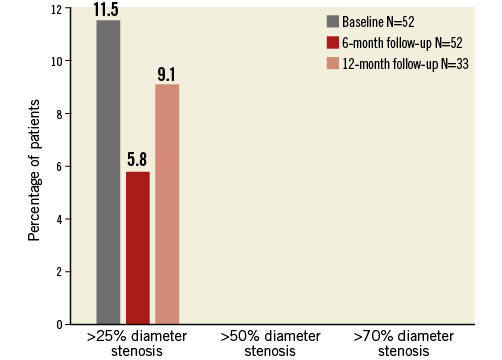
Figure 2. Percentage of patients with renal artery stenosis grade greater than 25%, 50% and 70% at baseline, six and 12 months.
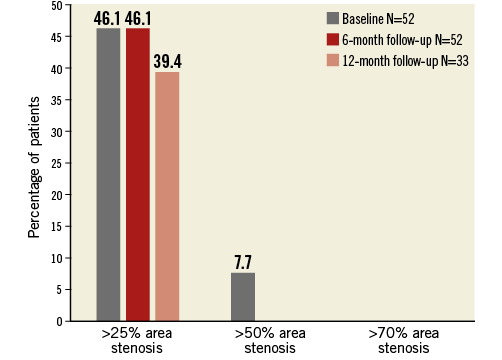
Figure 3. Percentage of patients with renal artery percentage area stenosis greater than 25%, 50% and 70% at baseline, six and 12 months.
Comparing the differences in late loss in vessels treated with a balloon-based system (Paradise, Vessix and OneShot) versus the Symplicity Flex non-balloon-based system did not reveal any significant differences (p=NS for all) (Figure 4).
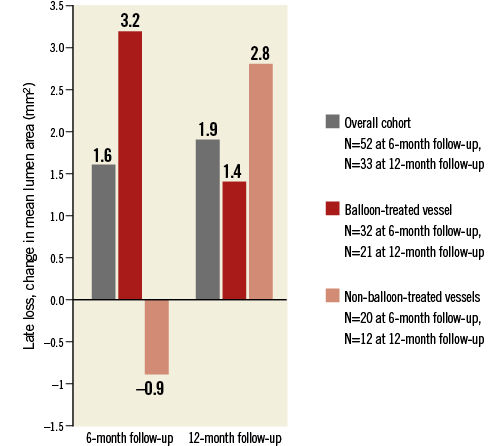
Figure 4. Change in mean lumen area from baseline to six and 12 months, respectively, in patients treated with balloon-based vs. non-balloon-based devices.
Intravascular imaging using optical coherence tomography directly post procedure revealed dissections in 6/19 vessels (32%) and thrombus in 13/19 vessels (68%). There was no correlation between these post-procedural signs of vascular traumata and luminal narrowing at six or 12 months.
Renal function slightly decreased over time. Mean baseline eGFR was 82.4 ml/min and decreased to 77 ml/min/1.73 m2 at six months and 74.9 ml/min/1.73 m2 at 12 months (p-value=0.013). Although there was no correlation between late loss and change in renal function at six or 12 months, a clear correlation was seen between contrast volume used periprocedurally and renal function decline over time. No new cases of renal impairment (eGFR <60 ml/min) were observed.
Discussion
Using quantitative MRA software we demonstrated an absence of renal artery stenosis at six and 12 months post procedure. More specifically, no decrease in renal artery dimensions post RDN could be found at either six or 12 months, irrespective of the device used.
The present study adds to previous observations reporting on the safety of RDN for three reasons. First, recent studies with a focus on follow-up of renal arteries six months after RDN used duplex ultrasound or occasionally CTA or MRA, and only focused on binary outcomes, namely the presence of stenosis or not. Using recently validated dedicated quantitative MRA software, we were able to report more accurately on the true vascular response of RDN13. Second, our study was not limited to the use of a single RDN device. Instead, the vascular response to treatment with both balloon-based and non-balloon-based devices as well as radiofrequency versus ultrasound devices was assessed. Third, we were able to extend the safety findings reported at six months to one year.
The need for a more precise method to assess renal artery dimensions was driven by the findings from several recent studies assessing the direct consequences of RDN on renal artery integrity11,15,16. Using dedicated intravascular imaging techniques such as IVUS and OCT, a significant proportion of renal arteries proved to be left with dissections, thrombus and oedema, mostly not directly visible on standard post-procedural angiography. Although in the vast majority of the cases these findings were not linked to direct clinical consequences, the effect on long-term vascular integrity and perhaps even renal function remains elusive. However, the importance of a careful assessment of long-term vascular safety has recently been underlined by the report of a European expert consensus document on future trial design in renal denervation17.
In a recently published validation study we demonstrated that renal artery dimensions can be accurately measured using CAAS MRA, with a good intraobserver and interobserver variability and a good correlation as compared to intravascular ultrasound13. Quantitative MRA facilitates non-invasive assessment of small changes in renal artery luminal dimensions and allowed us to study the correlation between periprocedural renal artery damage and long-term luminal narrowing. Although we hypothesised that a small, albeit significant change in luminal dimensions could be due to the cases in which the arterial lumen integrity was lost due to small dissections, thrombi and oedema, we were not able to demonstrate a correlation. Additionally, no differences were observed between luminal narrowing at follow-up and the use of any of the individual RDN devices used.
In our study, renal function deteriorated slightly over time. Although the technology has been previously hypothesised to improve renal function in patients with renal failure, the impact of the treatment on renal function over time has been disputed. While some single-arm studies have reported an improvement in renal function, others have demonstrated that renal function might deteriorate slightly over time18,19. We further hypothesised that luminal narrowing might be responsible for a decrease in renal function. However, no correlation was found between the change in luminal dimensions at both six- and 12-month follow-up and the change in eGFR. Further scrutiny of the pathophysiology of the renal function decline in our cohort revealed a clear correlation with contrast volume used during the procedure. Due to the learning curve in the initial cases performed at our site, together with the use of OCT imaging to assess renal artery integrity, contrast usage was substantially higher in the initial cases. It therefore appears unlikely that the RDN procedure itself causes a significant change in renal function over time.
Limitations
The sample size of our study was relatively small, which implies that the conclusions on the inter-device differences should be interpreted with caution. Unfortunately, several follow-up scans could not be analysed adequately due to the suboptimal quality of several MRAs with suboptimal harmonisation of contrast delivery and scan time and movement and breathing artefacts due to the need for a 20-second breath hold. Also, at the time of this study, not all patients reached the 12-month follow-up (n=2). Further research should focus on a larger study cohort and extended follow-up duration.
Conclusions
Quantitative MRA of patients treated with percutaneous renal denervation revealed no significant decrease in renal artery dimensions at both six- and 12-month follow-up. The lack of a decrease in renal artery luminal dimensions was irrespective of the arterial response to the individual devices used.
| Impact on daily practice Renal artery luminal dimension did not change after treatment with one of four different renal denervation devices when measured using dedicated quantitative MRA software. There was no correlation between post-procedural arterial damage as detected with invasive imaging and luminal narrowing at follow-up. The importance of a careful assessment of long-term vascular safety has recently been reported in a European expert consensus document for renal denervation. Using validated dedicated quantitative MRA software, our study has established that patients treated with RDN had no significant change in renal artery luminal dimensions up to 12 months post procedure. Device selection had no significant impact on our findings. |
Guest Editor
This paper was guest edited by Thomas Zeller, MD; Universitäts-Herzzentrum Freiburg, Bad Krozingen, Germany.
Conflict of interest statement
A. Karanasos has received research support from St. Jude Medical for OCT-related research. N. Van Mieghem is on the Advisory Board of Abbott Vascular. J. Daemen has received institutional research support from Boston Scientific, ReCor Medical, St. Jude Medical and Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

