
Abstract
Aims: With increasing attention to renovascular causes and targets for hypertension there arises a critical need for more detailed knowledge of renal arterial anatomy. However, a standardised nomenclature is lacking. The present study sought to develop a standardised nomenclature for renal anatomy considering the complexity and variation of the renal arterial tree and to assess the applicability of the nomenclature.
Methods and results: One thousand hypertensive patients underwent invasive selective renal artery angiography in nine centres. Further, renovasography was performed in 249 healthy swine as a surrogate for normotensive anatomy. Anatomical parameters were assessed by quantitative vascular analysis. Patients’ mean blood pressure was 168/90±26/17 mmHg. The right main renal artery was longer than the left (41±15 mm vs. 35±13 mm, p<0.001), but the left had a greater diameter (5.4±1.2 vs. 5.2±1.2 mm, p<0.001). Accessory renal arteries and renal artery disease were documented in 22% and 9% of the patients, respectively. Other than exhibiting a longer left main renal artery in uncontrolled hypertensives (+2.7 mm, p=0.034) there was no anatomical difference between patients with controlled and uncontrolled hypertension. Main renal artery mean diameter was smaller in patients with impaired kidney function (GFR <90 ml/min, left –0.5 mm, right –0.4 mm, both p<0.001).
Conclusions: Renal arterial anatomy differs between sides but shows no difference between patients with and without blood pressure control. Impaired GFR was associated with small main renal artery diameter.
Abbreviations
ACE: angiotensin-converting enzyme
CAD: coronary artery disease
DBP: diastolic blood pressure
GFR: glomerular filtration rate
QVA: quantitative vascular analysis
RAD: renal artery disease
SBP: systolic blood pressure
Introduction
Hypertension remains a major risk factor for the most significant cardiovascular events and is one of the most prevalent chronic conditions1. Despite its prevalence and the availability of safe and effective antihypertensive drugs, blood pressure control remains poor2. Renal artery stenosis (RAS) is a cause of secondary hypertension, especially among patients with other vascular atherosclerotic manifestations, and is closely associated with poor outcome3-6. Knowledge of the renal arterial anatomy appears crucial not only for a profound pathophysiological understanding of hypertension but also for the development of endovascular treatment options7,8. Morphometric data of the renal vascular tree in patients with hypertension9 and a consistent and standardised nomenclature are lacking10-12. The present study sought to develop a standardised nomenclature for renal anatomy considering the complexity and variation of the renal arterial tree and to assess the applicability of the nomenclature in 1,000 patients with hypertension undergoing renal arteriography.
Methods
Between March 2009 and June 2013, a total of 1,000 hypertensive patients underwent selective invasive renal angiography in eight European centres and one Australian centre (Appendix) in preparation for an invasive antihypertensive treatment. All participating patients provided written informed consent and local ethics committees approved the study. Eligible patients were ≥18 years old and had uncontrolled (systolic [SBP] and/or diastolic office blood pressure [DBP] ≥140/90 mmHg) or controlled hypertension (office blood pressure [OBP] at target with antihypertensive therapy). Patients routinely underwent a screening for secondary causes of hypertension, including duplex sonography or magnetic resonance/computed tomography angiography when clinically indicated3,6. Patients with haemodynamically significant renal artery stenoses ≥50% were excluded. All patients underwent a complete medical history check, physical examination and routine blood chemistry. Glomerular filtration rate (GFR) was assessed using cystatin C measurements. Attended OBPs were obtained with an automated oscillometric device (e.g., HEM-705 monitor; Omron Healthcare Inc., Vernon Hills, IL, USA) in concordance with the Joint National Committee VII guidelines13. The current antihypertensive medication was confirmed by direct questioning.
RENAL ANGIOGRAPHY AND QUANTITATIVE VASCULAR ANALYSIS (QVA)
All procedures were performed by experienced interventionalists (referring to an experience of ≥10 renal interventions per year). Non-ionic iodinated contrast agents were used in all procedures. Procedural data were recorded, and two experienced investigators blinded to patient characteristics assessed QVA using the CAAS II Research System (Pie Medical Imaging BV, Maastricht, the Netherlands).
ANATOMICAL PARAMETERS
Morphometric parameters such as minimum, mean and maximum diameter as well as length were documented for the main renal artery and in particular for the proximal (p), middle (m) and distal (d) segments, as previously described8. The division point in two or more consecutive branches of ≥3 mm in diameter defined the end of the main renal artery. Renal arteries other than the main renal arteries were defined as accessory renal arteries. These could be of similar size and penetrating the hilus or smaller and supplying a minor part of the kidney. Accessory renal arteries were evaluated regarding mean diameter and length. For further analysis, the largest calibre vessel of each side was determined. Moreover, the branches of 800 patients were analysed. The largest branches of each side were ascertained regarding length and diameter. Furthermore, the mean diameter and length were calculated. Renal artery disease included patients with non-significant renal artery stenosis (luminal narrowing 10-49%), prior renal angioplasty or stenting.
NOMENCLATURE OF RENAL ARTERIES
The nomenclature used for QVA was based on a three-letter code (Figure 1). All vessels proximal to the kidney’s parenchyma shadow with a mean diameter ≥3 mm were considered. The first letter indicated the laterality of the kidney (L for left and R for right kidney). The following two letters differentiated between main (MA, largest in diameter) and accessory renal arteries (AA). Subsequent branches were labelled with an additional B and, as with accessory renal arteries, numbered from cranial to caudal. Figure 2 gives an example of the application of the nomenclature for right renal arteries.
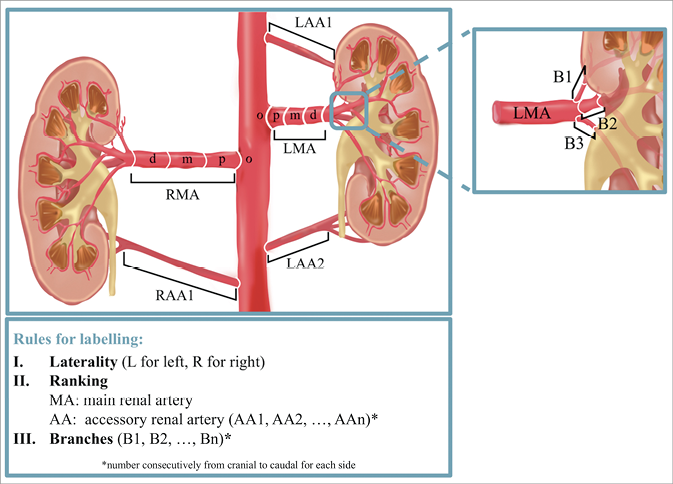
Figure 1. Nomenclature of renal arteries.
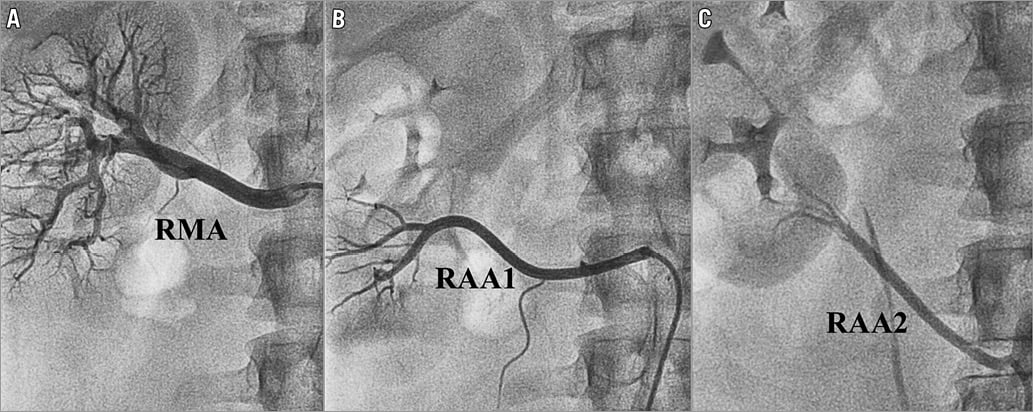
Figure 2. Invasive selective renal artery angiography. Renal artery anatomy of a 25-year-old man. The right main renal artery (RMA) and the upper accessory renal artery (RAA1) both arise from the aorta. The lower accessory renal artery (RAA2) originates from the right common iliac artery.
ANIMAL MODEL
Renal angiography, subsequent QVA and statistical analysis of the main renal arteries were performed in 249 healthy juvenile Yorkshire domestic farm swine at CBSET, Inc. (Lexington, MA, USA) in accordance with the Guide for the Care and Use of Laboratory Animals under an approved institutional animal care and use committee-approved protocol. The animal model was introduced as a surrogate for renal artery anatomy in normotensive humans. QVA was obtained using the Centricity Cardiology CA1000 Cardiac Review 2.0 software (GE Healthcare, Wauwatosa, WI, USA).
STATISTICAL ANALYSIS
Data management and all statistical analysis were carried out with IBM SPSS Statistics for Mac, Version 23.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean±standard deviation (SD) for continuous variables and as numbers (%) for categorical variables unless otherwise specified. Comparisons between groups were performed using Pearson’s χ2 test or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables where appropriate. A two-tailed p-value <0.05 was considered to be statistically significant.
Results
PATIENT POPULATION
Patients’ average age was 63.7±10.7 years; 57% were male with a body mass index (BMI) of 30.4±5.4 kg/m2. Coronary artery disease (CAD) and type 2 diabetes were prevalent in 270 (27%) and 375 (38%) patients, respectively. Despite an average of 4.8±1.7 prescribed antihypertensive drugs, SBP and DBP was 168±26 mmHg and 90±17 mmHg, respectively. ACE inhibitors or angiotensin receptor blockers were prescribed in 80% (721/901) of the patients, calcium channel blockers in 70% (567/805), diuretics in 80% (643/805), aldosterone antagonists in 20% (159/804), beta-blockers in 79% (635/804), centrally acting sympatholytic agents in 51% (388/755), alpha-adrenergic blockers in 27% (201/755), and direct-acting vasodilators in 23% (181/803). The mean heart rate (HR) was 66.9±11.6 beats per minute (bpm). Only 123 patients (12%) achieved blood pressure control, while 862 patients (88%) had uncontrolled hypertension (Table 1).
RENAL VASCULAR ANATOMY
On average, the right main renal artery was longer than the left main renal artery (+6.6 mm, p<0.001) (Figure 3A), whereas the left main renal artery was of slightly greater diameter (+0.2 mm, p<0.001) (Table 2, Table 3, Figure 3B). Main renal artery diameters were similar in patients with uncontrolled and controlled hypertension (left p=0.641, right p=0.615). Patients with uncontrolled hypertension had longer left main renal arteries (+2.7 mm, p=0.034), whereas the right main renal artery length did not differ. Patients’ age did not correlate with mean main renal artery diameter (left r=–0.215 and right r=–0.200, both p<0.001) or length (left r=0.093, p=0.003 and right r=0.102, p<0.001). When patients were grouped by baseline GFR values, lower GFR was associated with smaller main renal artery diameters (Figure 4). In patients with GFR <30 ml/min/1.73 m2, right and left main renal artery diameters were smallest when compared to patients with higher GFR values.
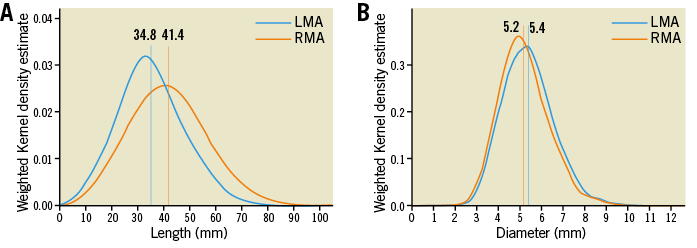
Figure 3. Main renal arteries. Length (A) and diameter (B) of the main renal arteries. Verticals indicate the mean value. LMA: left main renal artery; RMA: right main renal artery
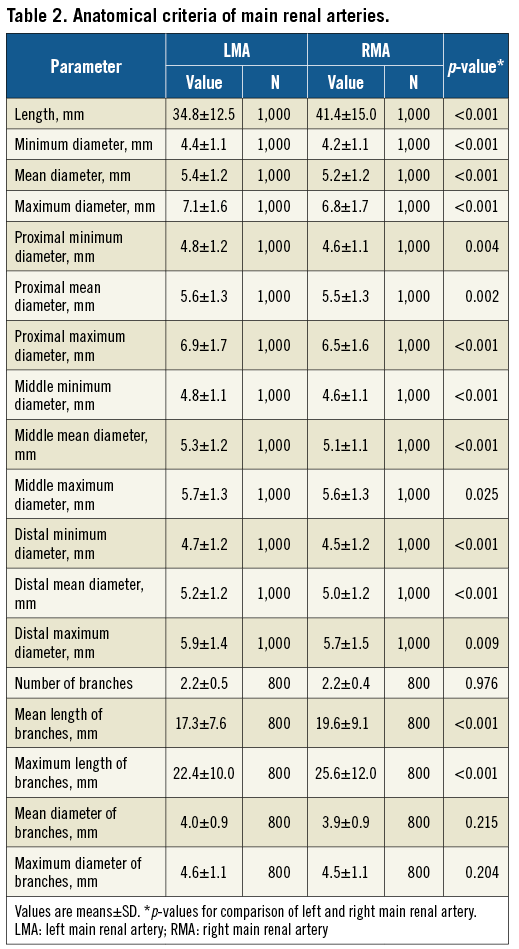
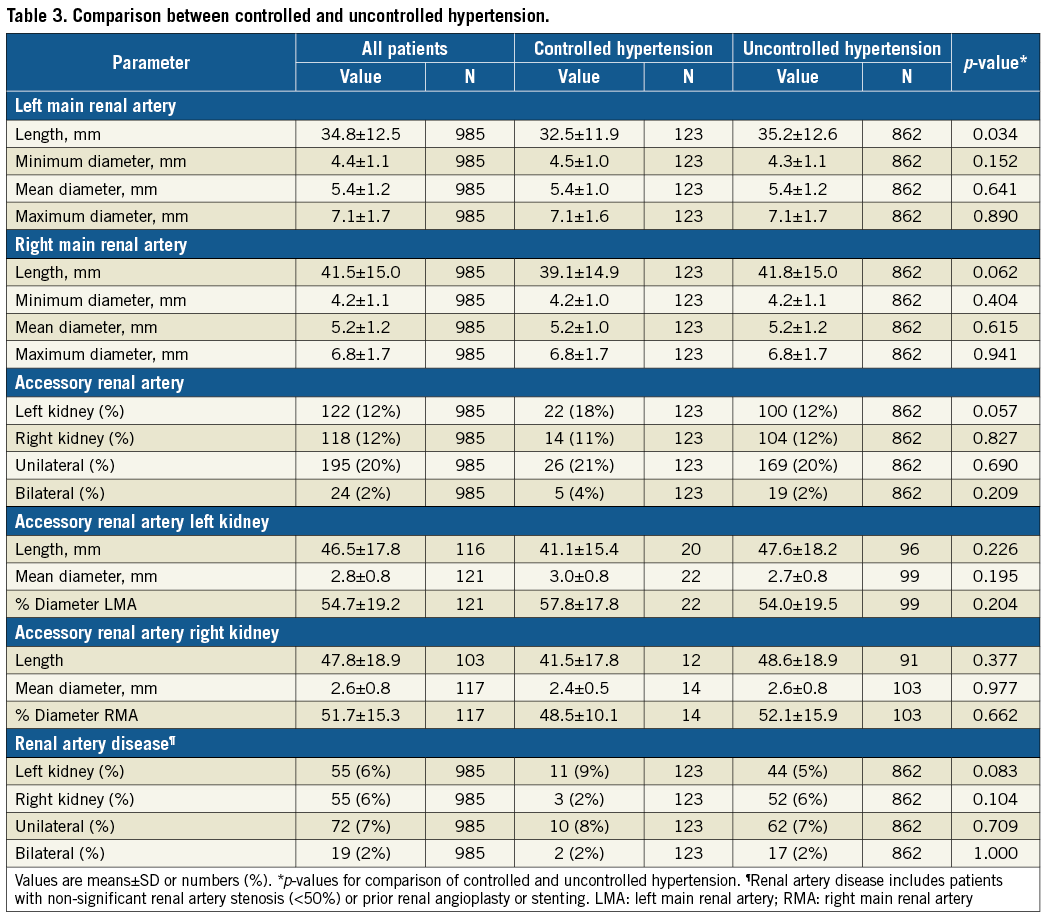
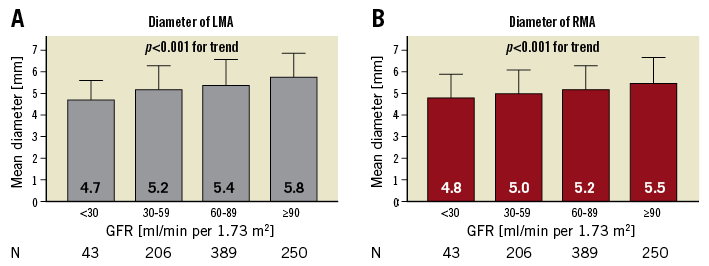
Figure 4. Mean diameter grouped by baseline GFR. Comparison of left (A) and right main renal artery (B) mean diameter when grouping patients by baseline GFR. p-values are comparison between groups. GFR: glomerular filtration rate; LMA: left main renal artery; RMA: right main renal artery
Accessory renal arteries were present unilaterally in 197 (20%) and bilaterally in 24 (2%) patients. In male patients, the prevalence of unilateral and bilateral accessory renal arteries was higher compared to female patients (unilateral p<0.001; bilateral p=0.009). The prevalence of accessory renal arteries differed neither between sides (p=0.681) nor between patients with uncontrolled and controlled hypertension (p=0.397), respectively. The mean diameter of the left accessory renal artery was greater than the diameter of the right accessory renal artery (+0.2 mm, p=0.019) whereas the lengths were similar (p=0.595). Patients with multiple renal arteries had longer left and right main renal arteries when compared to patients with solitary renal arteries (left +5.2 mm, right +6.9 mm, both p<0.001), whereas the mean main renal artery diameter (left p=0.151, right p=0.142) and the presence of renal artery disease (RAD) (left p=0.694, right p=0.553) were similar in both groups.
The branches of the left and right main renal arteries did not differ in terms of mean and maximum diameter (p=0.215 and p=0.204), but the branches of the right main renal artery were longer (mean length +2.3 mm, p<0.001; maximum length +3.2 mm, p<0.001).
RAD was diagnosed in 91 (9%) patients (Table 3). In comparison to patients without RAD, patients with RAD were older (66.6±10.7 vs. 64.4±10.7 years, p=0.003), had a higher prevalence of diabetes (51% vs. 37%, p=0.010) and CAD (37% vs. 27%, p=0.044), a lower DBP (87±16 mmHg vs. 90±17 mmHg, p=0.041) and a lower heart rate (64±9 bpm vs. 67±12 bpm, p=0.022), whereas SBP (167±22 mmHg vs. 168±26 mmHg, p=0.668) and pulse pressure (PP) (79±18 mmHg vs. 78±21 mmHg, p=0.309) were similar in both groups.
RENAL VASCULAR ANATOMY IN PORCINE MODEL
In 249 normotensive juvenile swine, the right main renal artery was longer than the left (+6.7 mm, p<0.001) whereas the left main renal artery was of greater diameter (+0.13 mm, p<0.001).
Discussion
The renal vascular anatomy typically shows a broad interindividual variety in the general population11, necessitating a standardised nomenclature. To the best of our knowledge, no accepted nomenclature for renal angiograms has been validated practically thus far11,14. We introduced a nomenclature which grasps the complexity of renal arterial anatomy and can be applied for clinical and research purposes. In addition, we implemented the nomenclature by analysing the renal vascular tree of 1,000 individuals with hypertension and subsequently introduced the animal model as a surrogate for renal artery anatomy in normotensive humans. The major findings were that i) accessory renal arteries are more common in men than in women, ii) blood pressure control correlates neither with morphological parameters nor with the presence of accessory renal arteries, and iii) low GFR is associated with small diameters of main renal arteries.
The liver forces the right kidney to be lower, more medially displaced and smaller than the left. The renal arteries arise from the lateral aspect of the aorta and, because the right artery passes posterior to the inferior vena cava, it is longer than the left. Indeed, our findings confirm these observations9,15. We add, however, that the left main renal artery is of larger diameter. Our data also suggest that accessory renal arteries are associated with longer main renal arteries, in contrast to previous studies that described solitary renal arteries as longer9. The main renal artery mean diameter was not affected by the presence of uncontrolled hypertension or accessory renal arteries. In the former respect, previous studies have reported inconclusive findings. Whereas Palmieri et al also found no difference between the diameter of main renal arteries with and without accessory renal arteries9, a small study using computed tomography documented main renal arteries to be smaller in the presence of accessory renal arteries16. Because our patient group consisted almost exclusively of patients with hypertension, we introduced an animal model as a surrogate for normotensive patients. Although the porcine renovascular anatomy is very similar in size to that of humans17, the renal artery length and diameter (not the proportions) may not be exactly translated to a human population. However, the data of the human and the porcine renal angiography both show the same proportions in size between right and left main renal arteries and may therefore allow drawing conclusions in a general population.
Our data also showed a positive relationship between main renal artery mean diameter and renal function as measured by GFR. Several pathophysiological mechanisms should be considered. Small renal diameters, especially in relation to renal mass, can cause an increase in renal sympathetic nerve activity18. Elevated renal sympathetic nerve activity increases renin secretion resulting in vasoconstriction and a subsequent decrease in renal blood flow (RBF) and GFR18,19. Therefore, small renal diameters may both be a consequence of reduced renal blood flow and reflect higher sympathetic tone which can potentially be affected by the means of renal denervation20. In the long term, flow-mediated decreases in shear stress may trigger endothelium-dependent inward arterial remodelling, leading to a narrowing of the renal arteries21. The association between GFR and main renal artery diameter is in line with findings in patients with renal artery stenosis, where small and minimal reference diameters were associated with low GFR and resistant hypertension22.
Accessory renal arteries were identified in 22% of the patients. Two extensive meta-analyses calculated a slightly higher prevalence of 23.3% and 28.2% for accessory renal arteries, respectively11,14. We documented accessory renal arteries to be more common in males than in females, whereas previous studies provide inconclusive evidence of a sex-specific difference9,11. However, the general role of accessory renal arteries in the development of hypertension remains elusive. A recently published study on the importance of accessory renal arteries for non-response to renal denervation showed accessory renal arteries to be overrepresented in resistant hypertensives and non-responders23. The authors argue that insufficient focal perfusion due to a mismatch between arterial perfusion and renal mass may result in an increased renin secretion23. A review of magnetic resonance angiography data suggests that accessory renal arteries are a vascular anomaly rather than an anatomical cause of hypertension24.
The overall prevalence of 9% for RAD (stenosis <50% or prior intervention) was higher compared with two previous studies analysing patients undergoing diagnostic cardiac catheterisation25,26. This may be related to the underlying hypertension in the present cohort. Patients with significant renal artery stenosis were excluded a priori from renal angiography to minimise selection bias, but several studies have shown that the prevalence of renal artery stenosis is higher among patients with uncontrolled hypertension (i.e., 15-40%) compared to hypertension in general6.
Limitations
As with all studies, the limitations of our work should be acknowledged. We aimed to perform quantitative analyses of renal artery anatomy in a large and representative cohort of 1,000 individuals; the sample size, however, was not based on an a priori power analysis. Although renal angiography of healthy swine showed similar results to our human patient group which consisted almost exclusively of patients with hypertension, many of whom lacked blood pressure control, the results may not be translated to the general population17. Future studies using less invasive diagnostic modalities such as magnetic resonance angiography27 are needed to compare healthy individuals with those affected by hypertension. Selective invasive renal angiography primarily provides two-dimensional images which may have reduced the accuracy of our measurements. The use of local vasodilators prior to imaging was at the interventionalists’ discretion. Thus, the extent of vascular tone may also have affected the measurements. Although the number of antihypertensive drugs prescribed was documented for all patients, detailed information was missing in some. The study did not aim to analyse subgroups of different antihypertensive drugs.
Conclusions
Renal arterial anatomy differs significantly when comparing renal arteries by side or sex but not when comparing patients with hypertension with and without blood pressure control. Further, accessory renal arteries are more common among men than women. Impaired renal function measured by GFR is associated with small main renal artery mean diameter.
| Impact on daily practice With increasing attention to renovascular causes and targets for hypertension there arises a critical need for more detailed knowledge of renal arterial anatomy. We proposed a new intuitive nomenclature which meets the requirements of the complex renal arterial anatomy and implemented it by analysing the renal vascular tree of 1,000 people with hypertension. Renal arterial anatomy differs significantly between sides and genders, but not when comparing patients with hypertension with and without blood pressure control. |
Appendix. Study collaborators
M. Bergmann, Cardiologicum Hamburg, Hamburg, Germany; E. Blessing, SRH Klinikum Karlsbad-Langensteinbach, Karlsbad, Germany; B. Cremers, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Universität des Saarlandes, Homburg/Saar, Germany; D. Hering, Baker IDI Heart and Diabetes Institute, Melbourne, Australia, and Dobney Hypertension Centre, University of Western Australia-Royal Perth Hospital Unit, Perth, Australia; L. Kaiser, Abteilung für Elektrophysiologie, Herzzentrum, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; S. Kulenthiran, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Universität des Saarlandes, Homburg/Saar, Germany; H. Nef, Medizinische Klinik I, Abteilung für Kardiologie und Angiologie, Universitätsklinikum, Gießen, Germany; E. Noory, Angiologie, Universitätsherzzentrum, Bad Krozingen, Germany; I. Sudano, Kardiologie, Universitäres Herzzentrum, Universitätsspital, Zürich, Switzerland; B. Vogel, Medizinische Klinik III, Universitätsklinikum Heidelberg, Heidelberg, Germany.
Acknowledgements
We would like to thank Jeannette Zimolong, Kathrin Gaspard, Lucas R. Daurella, and Anna-Maria Spognardi for excellent technical assistance.
Conflict of interest statement
F. Mahfoud, C. Ukena, and M. Böhm are supported by the Ministry of Science and Economy of the Saarland. M. Böhm and F. Mahfoud are supported by the Deutsche Forschungsgemeinschaft (SFB/Transregio 219) and the Deutsche Gesellschaft für Kardiologie. F. Mahfoud is supported by the Deutsche Hochdruckliga. M. Schlaich is supported by an NHMRC Senior Research Fellowship. E. Edelman is funded in part by a grant from the US National Institutes of Health (R01 GM 49039). All authors except L. Lauder, A. Tzafriri, E. Edelman, and B. Scheller received scientific support and speaker’s honoraria from Medtronic. F. Mahfoud received scientific support and speaker’s honorarium from ReCor. The other authors have no conflicts of interest to declare.

