Abstract
Background: The radial artery is recommended by international guidelines as the default vascular access in patients with acute coronary syndromes (ACS) managed invasively. However, crossover from radial to femoral access is required in 4-10% of cases and has been associated with worse outcomes. No standardised algorithm exists to predict the risk of radial crossover.
Aims: We sought to derive and externally validate a risk score to predict radial crossover in patients with ACS managed invasively.
Methods: The derivation cohort consisted of 4,197 patients with ACS undergoing invasive management via the randomly allocated radial access from the MATRIX trial. Using logistic regression, we selected predictors of radial crossover and developed a numerical risk score. External validation was accomplished among 3,451 and 491 ACS patients managed invasively and randomised to radial access from the RIVAL and RIFLE-STEACS trials, respectively.
Results: The MATRIX score (age, height, smoking, renal failure, prior coronary artery bypass grafting, ST-segment elevation myocardial infarction, Killip class, radial expertise) showed a c-index for radial crossover of 0.71 (95% CI: 0.67-0.75) in the derivation cohort. Discrimination ability was modest in the RIVAL (c-index: 0.64; 95% CI: 0.59-0.67) and RIFLE-STEACS (c-index: 0.66; 95% CI: 0.57-0.75) cohorts. A cut-off of ≥41 points was selected to identify patients at high risk of radial crossover.
Conclusions: The MATRIX score is a simple eight-item risk score which provides a standardised tool for the prediction of radial crossover among patients with ACS managed invasively. This tool can assist operators in anticipating and better addressing difficulties related to transradial procedures, potentially improving outcomes.
Introduction
Vascular access for coronary diagnostic and interventional procedures is typically obtained via the radial or femoral artery. Radial access is currently recommended by European and American professional societies as the default approach1,2 in view of substantial evidence showing advantages over femoral access in terms of clinical outcomes, quality of life, and costs3,4,5,6. In a non-negligible proportion of cases, however, transradial intervention may be hampered by unfavourable upper limb and aortic arch anatomy, resulting in a more demanding procedure and ultimately in the decision to switch to femoral access – radial crossover. In contemporary acute coronary syndrome (ACS) cohorts, radial crossover has been reported in approximately 8% of cases7,8,9, though this rate varies according to patient characteristics and the centres’ expertise3,4,5,9,10,11,12,13,14. The occurrence of radial crossover is undesirable since it has been associated with patient discomfort, increased radiation exposure, delayed revascularisation, and worse outcomes compared with successful radial access – particularly in ST-elevation myocardial infarction (STEMI) where prompt reperfusion is warranted7,8,9,10,11. Additionally, radial access failure and subsequent crossover to femoral access have been shown to abolish the bleeding benefit offered by the radial over the femoral artery, underlining the importance of initial access-site selection7,15. The upfront identification of patients at high risk of radial crossover could allow operators to anticipate, prevent, and/or overcome technical difficulties, and ultimately improve patient care and outcomes15. Two risk scores have been developed to predict radial crossover in patients undergoing elective and/or urgent percutaneous coronary intervention (PCI) from a single high-radial-volume centre9,12. However, these algorithms cannot be applied in centres with low-to-intermediate radial expertise, and their generalisability remains unclear since they have not been externally validated. Thus, no standardised and validated tool exists to predict the risk of radial crossover in patients invasively managed for ACS.
We created a novel risk score for the prediction of radial crossover in patients with ACS undergoing invasive management from the Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX) trial. The score was externally validated in two independent ACS cohorts from two large randomised trials.
Methods
STUDY DESIGN AND POPULATION
MATRIX (ClinicalTrials.gov, NCT01433627) was a programme of three nested, randomised, multicentre trials4. The MATRIX-Access trial compared radial versus femoral access in 8,404 patients with ACS, with or without ST-segment elevation, who were about to undergo invasive management and where the interventional cardiologist was willing to proceed via radial or femoral access and was expert in both (i.e., at least 75 coronary interventions performed, and at least 50% of interventions in ACS via the radial access during the previous year) (Supplementary Figure 1). Patients were randomised to radial or femoral access before starting coronary angiography using a web-based system. Access-site management was at the discretion of the treating physician16. Bivalirudin was infused consistently with approved labelling. Heparin was given at 70-100 units/kg or 50-70 units/kg if glycoprotein IIb/IIIa inhibitors were administered. All outcomes were centrally adjudicated by an independent clinical events committee blinded to treatment allocation. The trial was approved by the institutional review board at each centre, and patients gave written informed consent.
OUTCOMES
The primary endpoint was the occurrence of radial crossover at the index procedure. Radial crossover was defined as a failure to either start or complete coronary angiography or intervention via radial access and subsequent crossover to femoral or brachial access. All clinical and procedural variables included in the analysis were prospectively collected.
VALIDATION COHORTS
External validation of the score was done in two independent cohorts from the randomised multicentre RadIal Vs femorAL access for coronary intervention with ACS undergoing invasive management (RIVAL)3 and Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome (RIFLE-STEACS)5 trials. The RIVAL trial (ClinicalTrials.gov: NCT01014273) randomised 7,021 ACS patients, with or without ST-segment elevation, to receive invasive management by radial (n=3,507) or femoral (n=3,514) access. In the RIFLE-STEACS trial (ClinicalTrials.gov: NCT01420614), 1,001 patients with ST-segment elevation ACS were randomised to radial (n=500) or femoral (n=501) access for primary/rescue PCI. Enrolment criteria are listed in Supplementary Figure 1. In the validation cohorts, the score was calculated in all patients with complete information for score variables and assigned to each participant in a similar manner to the derivation cohort. Patients from the derivation and validation cohorts were enrolled in 240 sites from 33 countries (Supplementary Figure 2). To compare the novel score with previously proposed algorithms, we calculated the score proposed by Abdelaal et al12 (female sex, previous coronary artery bypass graft [CABG], cardiogenic shock) and the WRIST-CASE score9 (age, weight, creatinine, hypertension, prior PCI, cardiogenic shock, intra-aortic balloon pump, operator’s proportion of femoral interventions, intubation) (Supplementary Table 1). The primary endpoint for the score validation was the occurrence of radial crossover at the index procedure. Data in the validation cohorts were prospectively collected. The study protocols were approved by ethics committees in all participating institutions. Patients provided written informed consent.
STATISTICAL ANALYSIS
We studied the associations between possible predictors and radial crossover at index procedure with logistic regression in patients randomised to radial access from the MATRIX trial. Potential predictors of radial crossover were identified by consensus based on previous studies, clinical judgement, and prompt availability in the catheterisation laboratory, and selected at univariable analysis (p<0.10). Independent predictors were selected with multivariable backward selection (p<0.10). Linear predictor values were scaled and rounded to a score with integer values between 0 and 100. Discrimination was quantified by Harrell’s c-index17, and calibration was assessed comparing predicted and observed risks using calibration plots18. A cut-off point to identify patients at high versus low risk of radial crossover was selected to maximise the Youden index. C-indices, integrated discrimination improvement (IDI), and continuous net reclassification improvement (NRI)19 were computed to compare the performance of the MATRIX score with the score by Abdelaal et al and the WRIST-CASE score. Sensitivity analyses were performed to evaluate the score discrimination (i) after excluding patients in whom the randomly assigned radial access was not attempted by the operator in the derivation cohort, and (ii) using operator’s instead of centre’s proportion of radial intervention in the RIVAL trial, in which both operator’s and centre’s proportions of radial PCI were prospectively collected. The analyses were performed in accordance with the TRIPOD statement (Supplementary Table 2),20. Data were analysed using Stata 16.1 (StataCorp, College Station, TX, USA).
Results
Between October 2011 and November 2014, 8,404 patients were randomised in the MATRIX trial to receive radial (n=4,197) or femoral (n=4,207) access. Of those assigned to radial access, 183 (4.4%) underwent radial crossover at the index procedure, comprising 178 to femoral access and 5 to brachial access. Crossover occurred in 169 of 3,712 patients with initial right radial access and 14 of 485 patients with initial left radial access. Difficulties in establishing radial access accounted for 20.8% of cases. Radial failure occurred during coronary angiography or intervention in 50.3% and 13.1% of cases, respectively, mainly because of tortuosity or vasospasm. In 15.8% of patients, the operator did not attempt the randomly allocated radial access (Figure 1).
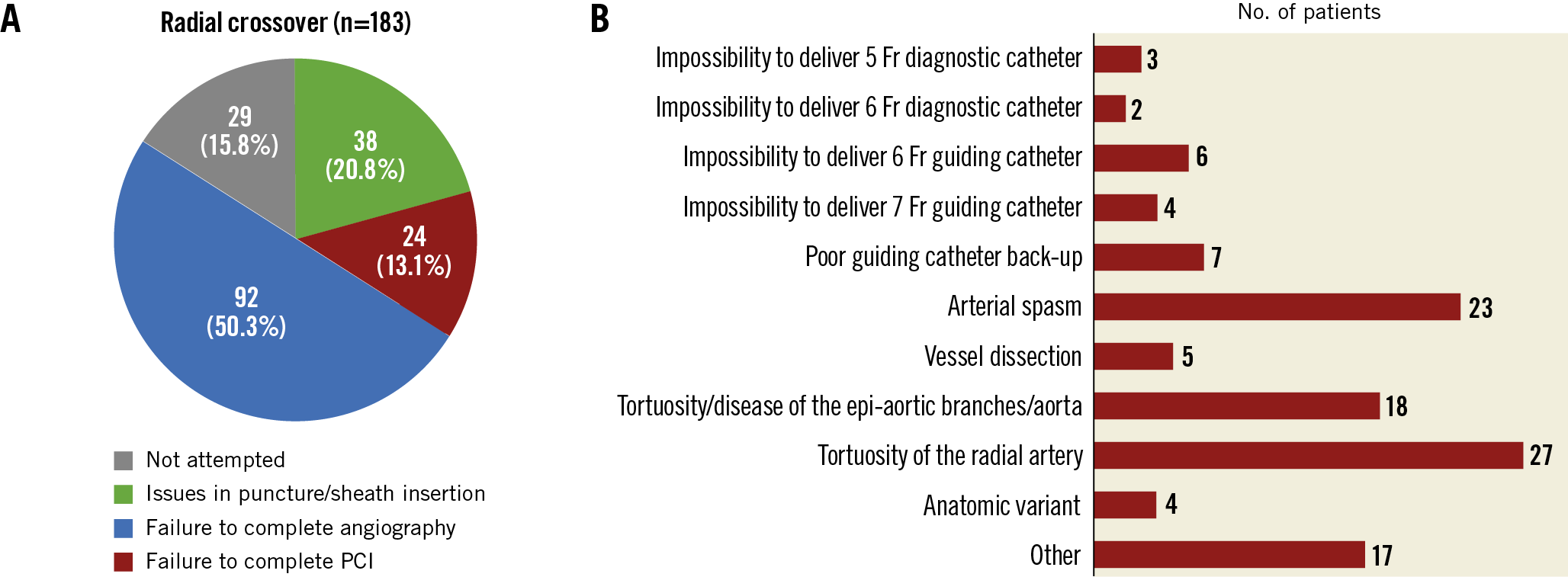
Figure 1. Radial crossover characteristics in the MATRIX trial. A) Reasons for radial crossover. B) Access-site issues causing radial crossover after successful sheath insertion. PCI: percutaneous coronary intervention
PREDICTORS OF RADIAL CROSSOVER AND SCORE DERIVATION
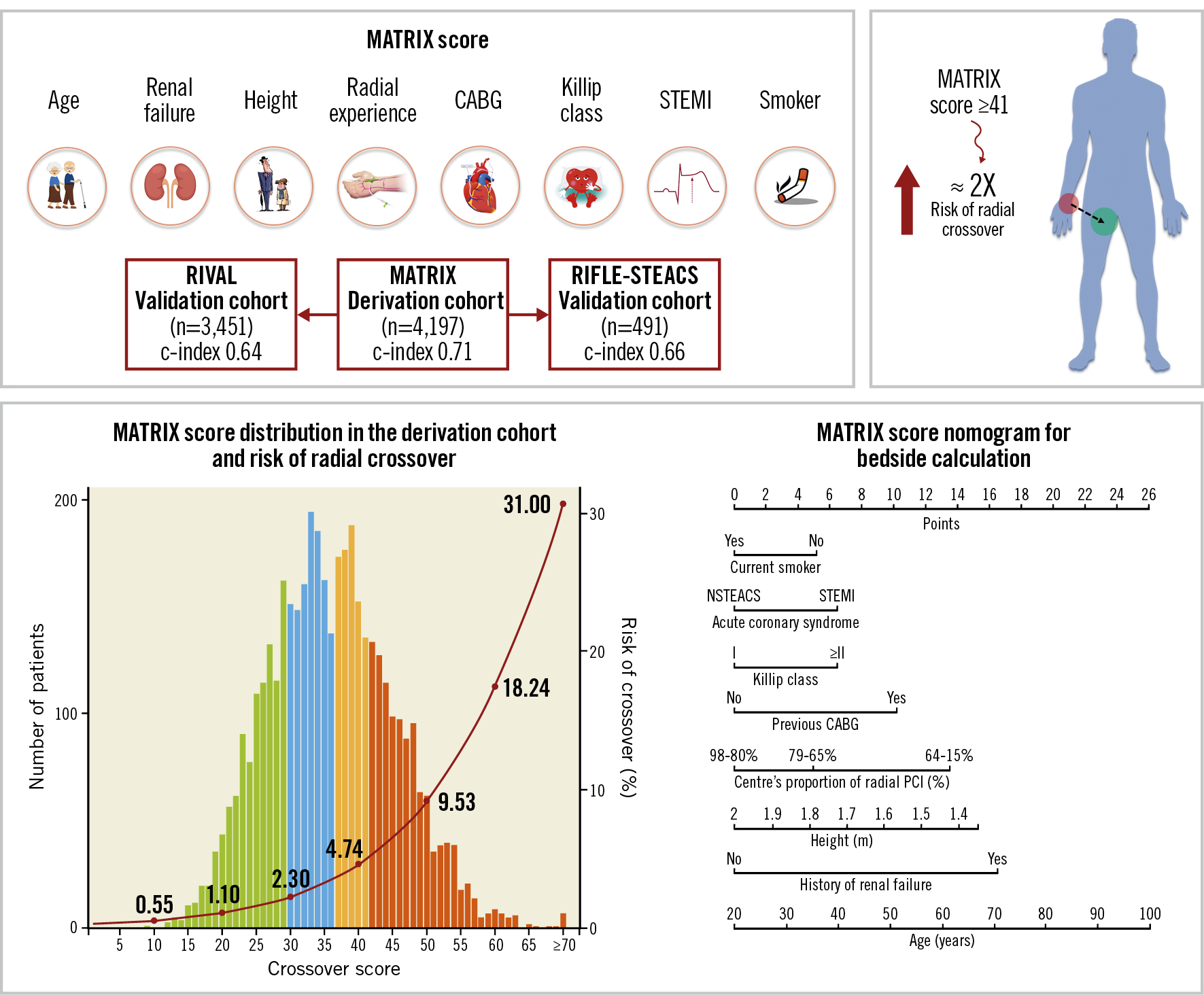
The derivation cohort included all 4,197 patients randomised to radial access in the MATRIX trial. Among potential predictors of radial crossover, those with a p-value of less than 0.10 at univariable analysis were included in the multivariable model. Eight independent predictors remained in the final model at a p-value of less than 0.10 (Table 1, Supplementary Appendix 1). From the final multivariable model, we developed an eight-item risk score (age, height, history of renal failure, previous CABG, current smoker, STEMI at presentation, Killip class, centre’s proportion of radial interventions – the MATRIX score), assigning points to each factor based on the magnitude of association of each predictor with radial crossover (Table 1, Supplementary Figure 3). The distribution of the MATRIX score in the derivation cohort, the risk of radial crossover by total score points, and a nomogram for score calculation are presented in the Central illustration. A web calculator is available online (www.matrixscore.org).
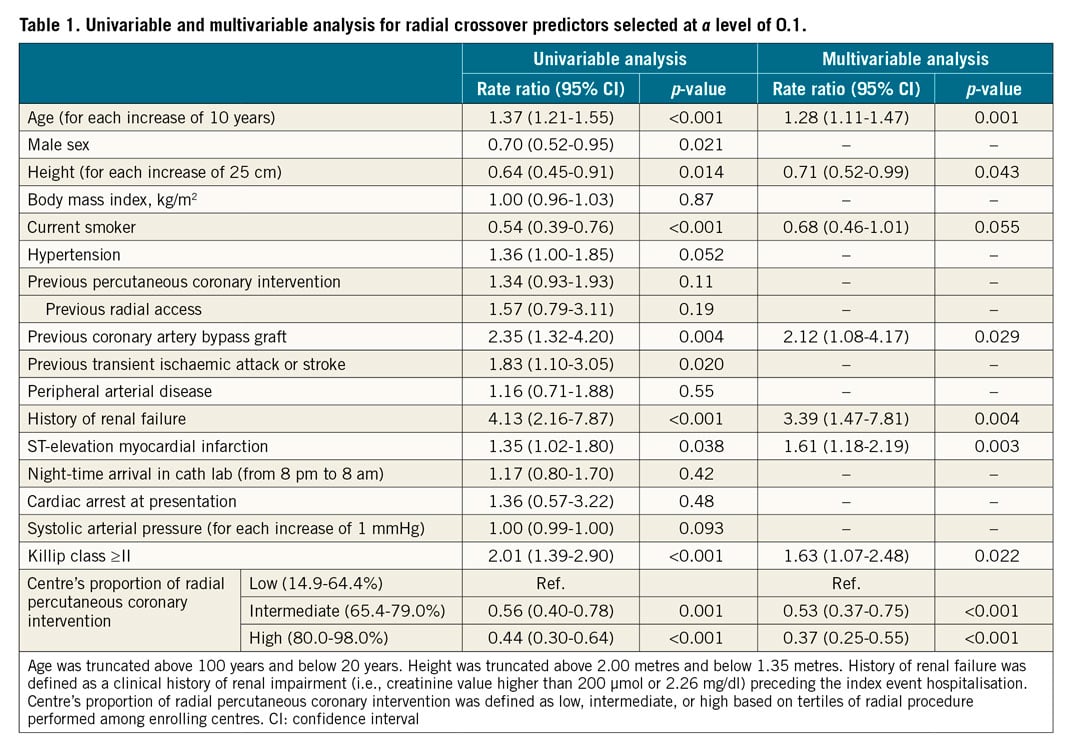
Central illustration. The MATRIX score for the prediction of radial crossover. The MATRIX score is a simple eight-item score to predict the risk of radial crossover in patients with ACS undergoing invasive management. A nomogram for bedside calculation is provided. The histogram refers to the MATRIX score distribution in the derivation cohort: green bars, the first score quartile of risk; blue bars, the second score quartile; yellow bars, the third score quartile; and orange bars, the fourth score quartile. The risk curve refers to the risk of radial crossover. At a cut-off of ≥41, the MATRIX score can select patients at higher risk of radial crossover compared with a lower score. ACS: acute coronary syndrome; CABG: coronary artery bypass graft; NSTEACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction
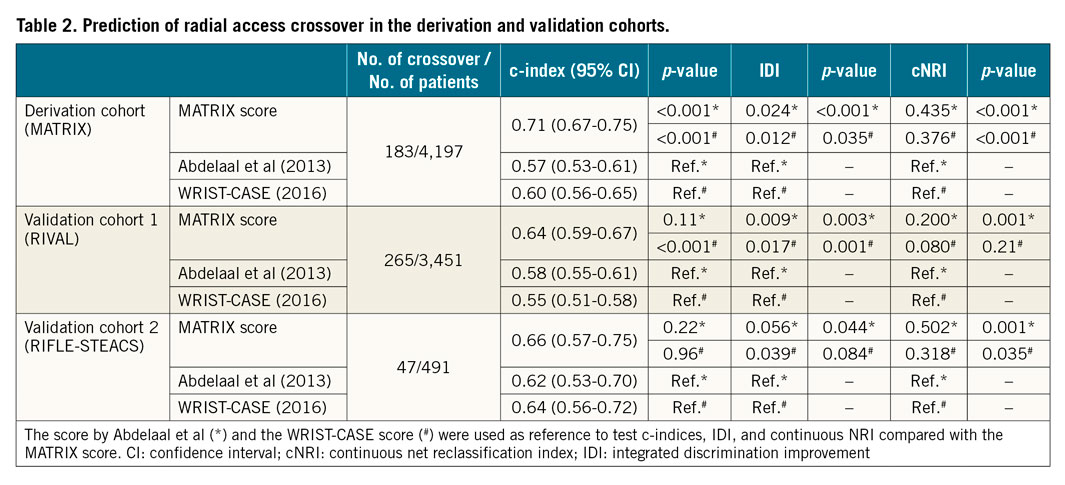
The MATRIX score showed a c-index of 0.71 (95% confidence interval [CI]: 0.67-0.75) for radial crossover and was well calibrated as indicated by the good agreement between predicted and actual risks (Figure 2). A cut-off of 41 points was selected to discriminate between patients at high versus low risk of crossover. Among patients randomised to radial access, 1,279 (30.5%) had a score ≥41 points and experienced a more than threefold higher risk of crossover compared with those at a lower score, showing an odds ratio (OR) of 3.67 (95% CI: 2.71-4.97; p<0.001) (Figure 3). These results remained consistent when right radial access (OR 3.90, 95% CI: 2.84-5.36; p<0.001]) and left radial access (OR 2.30, 95% CI: 0.77-6.61; p=0.14) were appraised separately. At very high score values (MATRIX score ≥70 points), patients had a predicted risk of radial crossover of 31% (Central illustration).
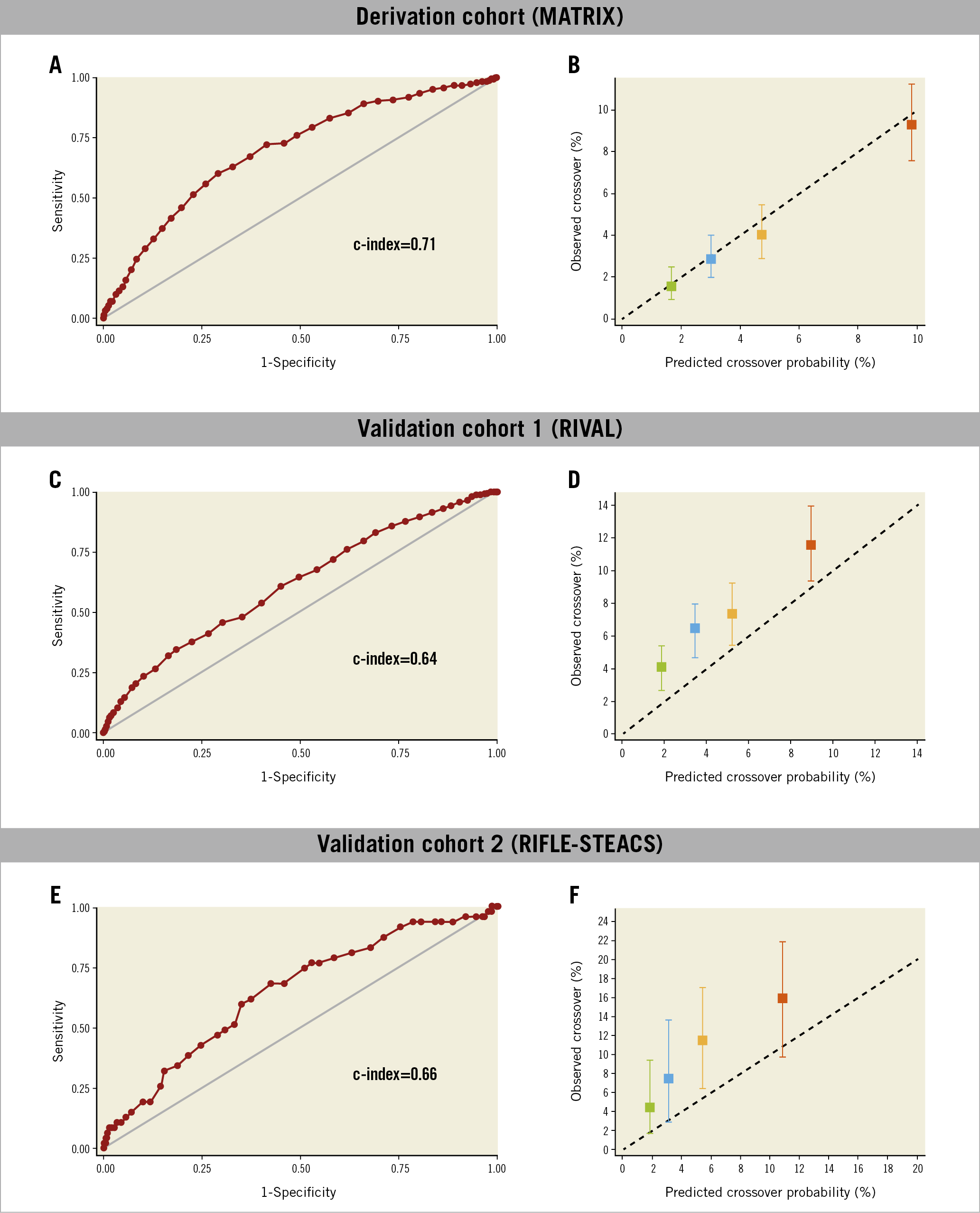
Figure 2. Receiver operating characteristic curve and calibration plot of the MATRIX score for the prediction of radial crossover in the derivation and validation cohorts. Receiver operating characteristic curves (A, C, & E). Calibration plots (B, D, & F). Quartiles of the patients by score are depicted including confidence intervals of risk estimates. The black dotted 45° line indicates perfect calibration (i.e., predictive value of the model perfectly matches patient’s actual risk). Any deviation above or below the 45° line indicates under- or over-prediction, respectively.
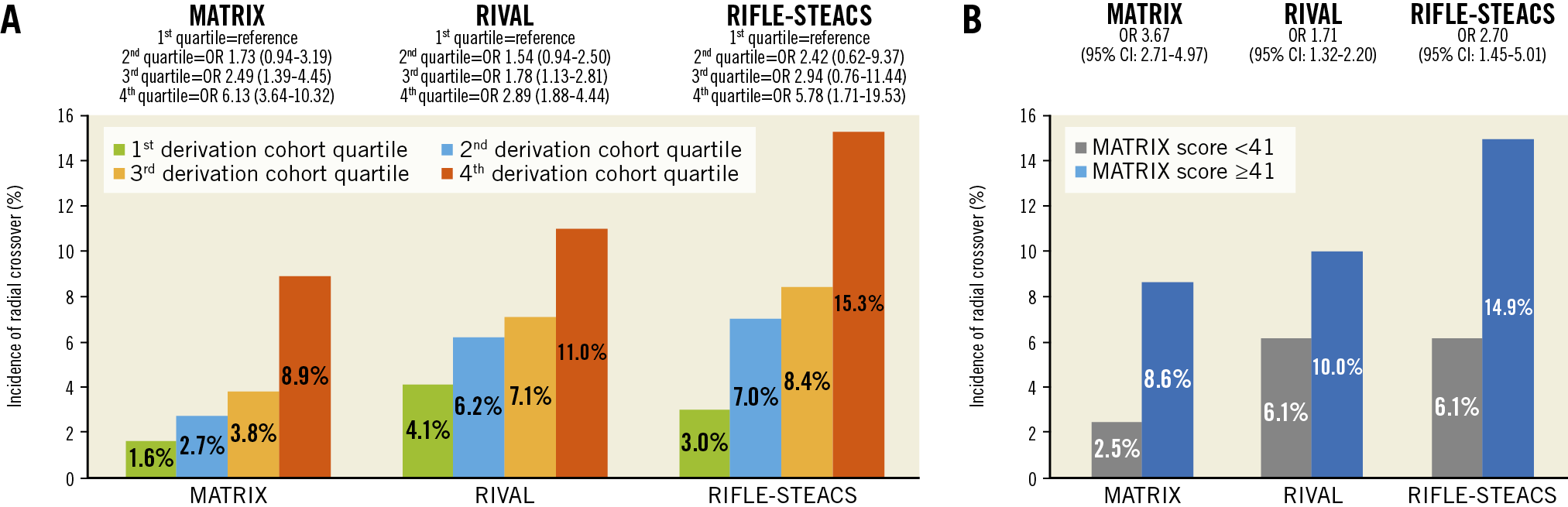
Figure 3. Cumulative incidence and odds ratio of radial crossover according to the MATRIX score in the derivation and validation cohorts. A) Risk of radial crossover stratified by MATRIX score in the derivation and validation cohorts using as cut-off points those separating quartiles in the derivation cohort. B) Risk of radial crossover stratified by MATRIX score at a cut-off point of 41 in the derivation and validation cohorts. CI: confidence interval; OR: odds ratio
EXTERNAL VALIDATION IN THE RIVAL AND RIFLE-STEACS TRIAL COHORTS
The MATRIX score was validated in 98.4% (n=3,451) and 98.2% (n=491) of the patients randomised to radial access with complete information for score calculation in the RIVAL and RIFLE-STEACS trials, respectively. The rate of radial crossover was 7.7% (265 events) in the RIVAL cohort and 9.6% (47 events) in the RIFLE-STEACS cohort. The c-index for radial crossover was 0.64 (95% CI: 0.59-0.67) in the RIVAL cohort, and 0.66 (95% CI: 0.57-0.75) in the RIFLE-STEACS cohort. The score maintained a consistent association between predicted probabilities and observed frequencies in the validation cohorts, although crossover risk was systematically underestimated in both external study cohorts (Table 2, Figure 2). At a cut-off ≥41, the score identified 1,304 patients (37.2%) in the RIVAL and 204 patients (40.8%) in the RIFLE-STEACS cohorts at increased crossover risk compared with those at a lower score (OR 1.71, 95% CI: 1.32-2.20; p<0.0001, and OR 2.70, 95% CI: 1.45-5.01; p=0.0029, respectively) (Figure 3).
PERFORMANCE COMPARED WITH PREVIOUS RISK SCORES
The score by Abdelaal et al showed a c-index for radial crossover of 0.57 (95% CI: 0.53-0.61) in the MATRIX, 0.58 (95% CI: 0.55-0.61) in the RIVAL, and 0.62 (95% CI: 0.53-0.70) in the RIFLE-STEACS cohorts. The c-index of the WRIST-CASE score was 0.60 (95% CI: 0.56-0.65), 0.55 (95% CI: 0.51-0.58), and 0.64 (95% CI: 0.56-0.72) in the MATRIX, RIVAL, and RIFLE-STEACS cohorts, respectively (Table 2).
The MATRIX score showed a superior discriminative ability according to the c-index compared with the score proposed by Abdelaal et al, with a non-significant effect in the RIVAL (p=0.11) and RIFLE-STEACS (p=0.22) cohorts. The discrimination offered by the MATRIX score was significantly superior to that provided by the WRIST-CASE score in the RIVAL (p<0.001), but not in the RIFLE-STEACS cohort (p=0.96) (Table 2).
The IDI and continuous NRI analysis showed improved risk stratification with the MATRIX score compared with previous scores, although differences were not always significant versus the WRIST-CASE score (Table 2).
SENSITIVITY ANALYSES
In the MATRIX trial, the randomly assigned radial access was not attempted by the operator in 29 cases. The median MATRIX score in these patients was 44 (interquartile range 38-48). After excluding these cases, the performance of the score in the derivation cohort remained consistent (c-index 0.69, 95% CI: 0.65-0.73).
When the score c-index was computed using operator’s instead of centre’s proportion of radial intervention in the RIVAL trial, the discrimination ability remained almost identical (c-index 0.63, 95% CI: 0.60-0.66).
Discussion
We developed the first externally validated score to predict the risk of crossover from radial access to mainly femoral access among ACS patients managed invasively. It consists of eight readily available items, namely age, height, smoking status, history of renal failure, prior CABG, Killip class, STEMI at presentation, and radial expertise. The generation data set consisted of the largest randomised trial comparing radial versus femoral access. The validation data sets included the second-largest trial with randomised vascular access and one of the largest randomised data sets focusing on STEMI3,5. The score ranges from zero to 100 arbitrary points and identifies – at a cut-off point of 41 – a sizeable proportion of patients, approximating 30% of the study cohorts, in whom crossover risk seems at least doubled and close to 10% in absolute terms. The MATRIX score is meant to assist operators in anticipating and potentially addressing difficulties in performing transradial procedures.
Our study does not offer insights into whether a primary femoral approach might be associated with similar or even improved outcomes compared to radial access in patients at high crossover risk. However, it provides for the first time a standardised tool to quantify crossover risks and inform operators, serving as a basis for future research. For instance, patients at high predicted risk of radial failure may be considered for more extensive use of ultrasound-guided radial artery cannulation to prevent issues in arterial puncture or sheath insertion (accounting for 20% of radial crossover cases in our cohorts). Also, before attempting radial access, a careful examination of the femoral arteries appears desirable if a high risk of crossover exists in order to plan safe alternative vascular access. Additionally, the upfront selection and/or prompt availability of procedural materials that can facilitate vascular navigation, reduce the need for catheter exchanges, and address possible transradial difficulties (e.g., hydrophilic sheaths, guidewires, and catheters, dedicated radial shapes)21 may be warranted in these cases. The MATRIX score may also serve as a benchmark and quality measure for centres and operators during and after the adoption of transradial interventions in daily practice. A stepwise approach to learning may be proposed based on the predicted risk of radial crossover, and patients with an increasing score may be treated gradually via the radial artery. The highest level of radial competency for institutions and operators may be established when patients at a high score and requiring complex procedures can be managed with timely and technically proficient control of coronary interventions. Finally, estimating the risk of crossover to the femoral access upfront may provide a unique asset during patient counselling and the informed consent process, especially for the growing number of patients who explicitly request radial access. Whether radial access should still be preferable in patients at high risk for radial crossover remains unclear and should be prospectively investigated in clinical studies.
The use of individual factors and risk scores to identify patients at risk of radial crossover has been proposed previously. However, prior studies have been flawed by several limitations, undermining the applicability of their conclusions, including small sample size and the absence of external validation9,10,11,12,13. The MATRIX score showed modest performance in two large independent validation cohorts of ACS patients. The higher discriminative ability in the RIFLE-STEACS trial compared with the RIVAL trial may reflect differences in study populations. For instance, in the RIVAL trial, patients with cardiogenic shock were excluded, and those with STEMI and advanced Killip class were more likely enrolled in high-radial-volume centres3. The score discrimination ability in terms of the c-index was relatively modest but at least similar to (or numerically higher than) previously proposed algorithms for radial crossover risk9,12 and other widely adopted cardiovascular and non-cardiovascular prediction models22,23. The c-index is a widespread measure of discrimination, which is however well known to be insensitive for assessing the clinical implications of prediction models24. As such, reliance on the c-index alone has significant limitations; other measures – such as calibration – should be considered. In this respect, the MATRIX score was well calibrated, as indicated by the good agreement between predicted and actual risks of radial crossover, and maintained a consistent effect in both validation cohorts.
The present analysis confirms the predictive value of well-known risk factors for radial failure, such as advanced age, previous CABG, and renal failure9,10,11,12,13. We featured the relevance of radial expertise, which emerged as a strong risk predictor in our model. Our findings are consistent with previous studies reporting an increase in the proficiency of radial access and a reduction in the rate of crossover as the radial expertise grows11,14,25. We observed that height has an independent association with radial crossover, which has been reported in some studies26,27 but not by others9,10,11,12,13. The predictive value of this variable has a sound rationale since a short height (or height loss due to senescence) is known to predispose to vascular tortuosity of the upper limb arteries28. Moreover, in patients with short stature, a small aortic root or short ascending aorta can hamper stable coronary cannulation during radial intervention, therefore increasing the risk of failure. In contrast with previous reports suggesting more difficulties for radial intervention among women11,12,21,27, female sex did not arise as an independent predictor of radial crossover in our model. Of note, two of the predictors included in the MATRIX score – advanced age and short height – frequently coexist in women29 and may potentially underpin this misconception. Finally, the presence of ongoing cardiac ischaemia and haemodynamic compromise – marked by STEMI presentation and advanced Killip class – were independently associated with increased risk of radial crossover, which is in agreement with previous observations9,12. Of importance, all the variables included in the novel score are readily available to the interventional team and do not imply any delay for data collection (i.e., no laboratory data required), which is essential for any novel algorithm proposed to improve ACS management.
Two risk scores for the prediction of radial crossover have been developed previously in the context of a single-centre experience. A first score was derived from 1,654 patients undergoing PCI (77% for ACS)12. The same group of investigators developed the WRIST-CASE score from 2,020 STEMI patients undergoing primary PCI9. The need for creatinine on admission for score calculation, which is not generally available at the time of intervention, limits its use in clinical practice. Moreover, the exclusion of patients with prior CABG precludes the application of the algorithm in this subset. As both models were derived from a single high-radial-volume institution without external validation, it remained unclear whether they apply to centres with low-to-intermediate radial expertise, which might benefit more from these tools. With respect to crossover risk prediction, our score proved at least as good as both scores, showing c-indices consistently numerically, even if not always statistically, superior. In addition, our findings seem to reflect the relatively low number of crossover cases in the RIFLE-STEACS cohort in which the superiority of the MATRIX score over the WRIST-CASE score could not be confirmed – in contrast with the comparative data observed within the generation and RIVAL data sets. Finally, at variance from our algorithm, neither of the two previously available scores offered a cut-off point for selecting patients at higher risk of crossover. Interestingly, the Youden index analysis identified at 41 the best score trade-off between sensitivity and specificity, which is consistent with the boundary between the third and fourth quartiles in the generation data set.
Limitations
Our analysis has several limitations. The discrimination ability of our score was modest. Future investigations should assess whether enlarged or modified sets of baseline covariates might improve discrimination. Our findings suggest that access crossover remains a rather technical aspect that is difficult to predict accurately upfront. Information on coronary anatomy and the complexity of the required intervention is probably critical in the decision making for crossover. However, angiographic characteristics are unknown before access-site selection and are not useful to improve crossover prediction. The score was able to risk stratify patients when initial right and left radial accesses were appraised separately, although the low number of patients in the latter group precludes definite conclusions. Information on the type of CABG surgery was not collected in the trial data sets, preventing more granular analyses. A minority of crossover cases followed the operator’s decision not to attempt radial access. Consistent with previous studies9,12, these cases were included to avoid case selection, and results remained consistent at sensitivity analyses excluding these patients. Radial expertise was appraised in the derivation cohort based on the centre’s experience. However, when the score c-index was computed using operator’s rather than centre’s proportion of radial PCI in the RIVAL trial, results remained entirely consistent, suggesting that such information can be used alternatively for the score calculation. Our score underestimated the crossover risk in the validation cohorts, probably because of the higher crossover rates observed in these data sets compared with the derivation cohort. The MATRIX score was generated and validated using data from clinical trials. The derivation and validation cohorts included unselected high-risk patients, and their clinical characteristics are consistent with those reported in contemporary registries30. However, the study exclusion criteria (i.e., peripheral vascular disease, end-stage renal disease) may still limit the applicability of the score in some specific settings. Whether the routine use of the MATRIX score could reduce the rate of radial crossover and improve procedural and/or clinical outcomes remains to be determined.
Conclusions
We developed the MATRIX score, a simple eight-item tool to predict the risk of radial crossover in patients with ACS undergoing invasive management. It is well calibrated and has modest discrimination at external validation analysis. At a cut-off point of ≥41, the MATRIX score identifies patients with an at least twofold higher risk of radial crossover compared with those at a lower score. The novel score may assist operators in anticipating and potentially better addressing issues with radial access in clinical practice and provides a new opportunity to investigate the risks and benefits of radial access versus direct femoral access in this high-risk patient subset.
|
Impact on daily practice The MATRIX score is the first standardised tool to predict the risk of radial crossover in patients with ACS undergoing invasive management. The novel score can identify, at a cut-off of ≥41 points, patients at high risk of radial crossover, and assist operators in anticipating and potentially addressing issues associated with transradial interventions in daily practice. Patients at high risk of radial failure may be considered for the use of specific techniques and materials that can facilitate radial artery cannulation and navigation (e.g., ultrasound-guided radial access, hydrophilic sheaths, guidewires, and catheters, dedicated radial shapes). The MATRIX score may also serve as a benchmark and quality measure for centres and operators during and after the adoption of transradial interventions in clinical practice. |
Funding
The MATRIX trial was sponsored by the Società Italiana di Cardiologia Invasiva (GISE, a non-profit organisation), which received grant support from The Medicines Company and Terumo. This substudy did not receive any direct or indirect funding.
Conflict of interest statement
S. Leonardi reports grants and personal fees from AstraZeneca, BMS/Pfizer, and Chiesi, and personal fees from Bayer, outside the submitted work. G. Gargiulo reports consultant fees from Daiichi Sankyo, outside the submitted work. P. Vranckx reports personal fees from AstraZeneca, Terumo, CSL Behring, Daiichi Sankyo, and Bayer Health Care outside the submitted work. E. Frigoli, D. Heg, and M. Branca are affiliated with CTU Bern, University of Bern, which has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organisations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. S. Windecker reports research and educational grants to the institution from Abbott, Abiomed, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, Cardiovalve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. He serves as unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments from pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. He is an unpaid member of the Pfizer Research Award selection committee in Switzerland. G. Biondi-Zoccai reports personal fees from InnovHeart, Meditrial, and Replycare, outside the submitted work. M. Valgimigli reports grants and personal fees from Abbott Vascular, personal fees from Chiesi, Bayer, CoreFlow, Opsens, Daiichi Sankyo, and Amgen, grants and personal fees from Terumo, personal fees from Alvimedica, grants from Medicure, grants and personal fees from AstraZeneca, personal fees from Biosensors, Idorsia, Bristol Myers Squibb, iVascular, Medscape and Vifor, outside the submitted work. G. Ando reports non-financial support from Terumo during the conduct of the study, personal fees and non-financial support from Bayer, Daiichi Sankyo, Bristol Myers Squibb-Pfizer, and Boehringer Ingelheim, personal fees from Menarini, AstraZeneca, Chiesi, and Biosensors, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

