
Introduction
The ASET (Acetyl Salicylic Elimination Trial) pilot study (NCT03469856) is designed to evaluate the hypothesis that a single antiplatelet therapy with prasugrel starting immediately after the procedure is feasible and safe in patients selected after successful percutaneous coronary intervention (PCI) with new-generation biodegradable polymer drug-eluting stent (DES) implantation.
Methods
STUDY DESIGN
The ASET pilot study is designed as a multicentre, single-arm, open-label, proof-of-concept pilot trial. Based on previous pilot studies with similar designs, a sample of 200 patients will be enrolled from 12 centres in Brazil with a stopping rule based on the occurrence of definite stent thrombosis1. If more than three cases of definite stent thrombosis occur following the index procedure until three-month follow-up, the patient recruitment will be terminated. All potential patients must be consented prior to undergoing any study-specific procedures.
PATIENT SCREENING BEFORE INDEX PCI
Patients requiring PCI for chronic stable angina or stabilised acute coronary syndrome (ACS) and having a SYNTAX score <23 prior to revascularisation will be screened and considered eligible for the study (Figure 1). Stabilised ACS fulfils the criteria of cardiac enzymes described in Supplementary Appendix 1. Inclusion and exclusion criteria are shown in Supplementary Appendix 1 and Supplementary Table 1.
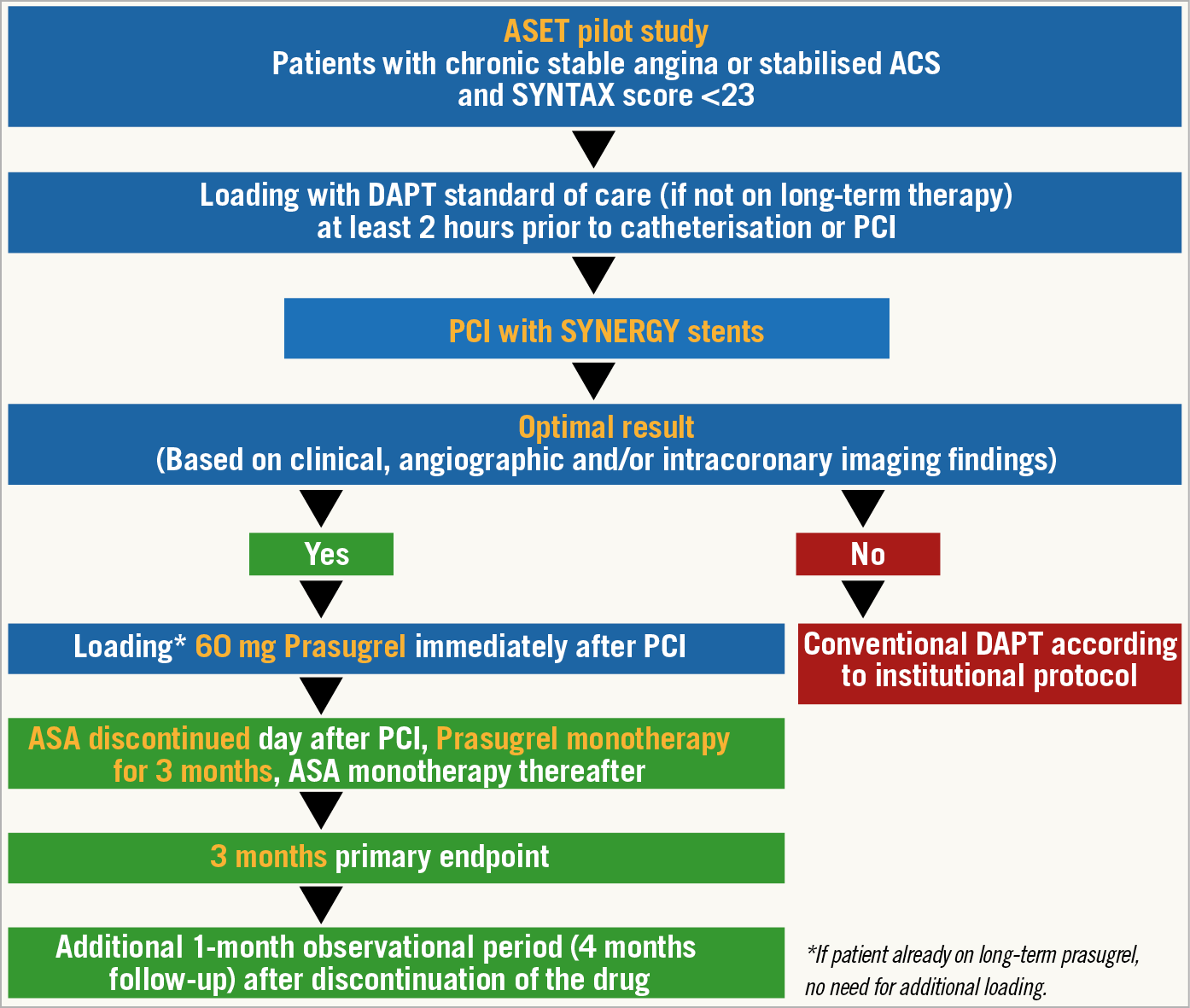
Figure 1. Flow chart of the ASET pilot study. ACS: acute coronary syndromes; ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention
INDEX PCI
The patients will be loaded with standard dual antiplatelet therapy (DAPT: aspirin 300 mg and clopidogrel 600 mg, unless patients are on long-term therapy) at least two hours prior to the index PCI.
The index PCI will be performed with the intention of achieving complete revascularisation of all vessels with at least 1.5 mm diameter showing a stenosis of 50% or more, as identified by the local interventional cardiologist. All target lesions must be exclusively treated with the SYNERGY™ stent (Boston Scientific, Marlborough, MA, USA).
The investigator should perform the procedure aiming to achieve optimal stent implantation according to local standard of care by angiography including quantitative coronary angiography and/or finding from intracoronary imaging (intravascular ultrasound or optical coherence tomography). Recommended criteria for optimal stent implantation are shown in Supplementary Figure 1 and Supplementary Table 2,2.
ENROLMENT AND FOLLOW-UP
After achievement of optimal stent implantation, patients will be enrolled in the study and loaded with prasugrel 60 mg in the cath lab, in line with international consensus on switching therapies3. Prasugrel 10 mg once a day will be continued as monotherapy for three months. After three months, prasugrel will be replaced by aspirin monotherapy or DAPT, according to local standard of care. When switching from prasugrel back to aspirin monotherapy only, a loading dose of aspirin is recommended and must be given in the hospital at the time of the three-month follow-up visit. Clinical follow-up with an office visit will be performed at three months and telephone contacts at one and four months.
STUDY ENDPOINTS
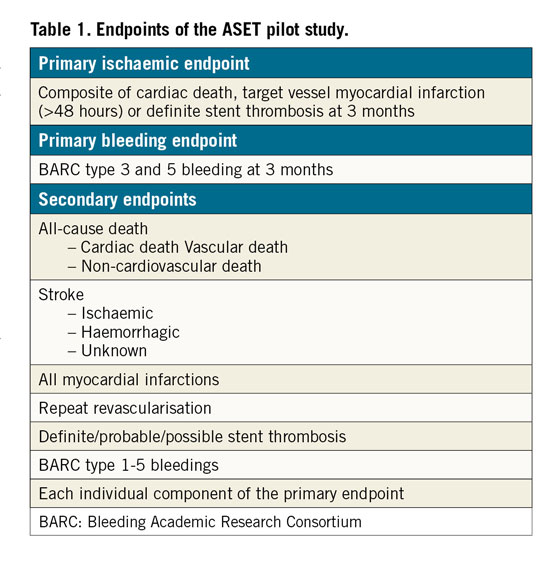
The study endpoints are listed in Table 1. The primary ischaemic endpoint is a composite of cardiac death, target vessel myocardial infarction (MI) (>48 hours, i.e., without periprocedural MI having occurred within 48 hours after the index procedure) or definite stent thrombosis at three months. The primary bleeding endpoint is any Bleeding Academic Research Consortium (BARC) type 3 and 5 bleeding at three months. Definitions of the study endpoints are described in Supplementary Appendix 1.
All endpoints will be independently adjudicated by the clinical events committee (CEC). An independent data safety and monitoring board (DSMB) will monitor the individual and collective safety of the patients in the study during the enrolment phase and up to three-month follow-up (primary endpoint).
Discussion
Regarding an aspirin-free strategy after DES implantation, currently two different types of evidence have been reported.
Firstly, in the AUGUSTUS trial, oral anticoagulation with a P2Y12 inhibitor demonstrated reductions in bleeding events without any trade-off in ischaemic events compared to oral anticoagulation with aspirin in patients with atrial fibrillation who had an ACS or had undergone PCI4.
Secondly, in the GLOBAL LEADERS trial, ticagrelor monotherapy following one-month DAPT failed to demonstrate superiority for a composite of all-cause mortality or new Q-wave MI at two years compared with aspirin monotherapy following 12-month DAPT5. However, two randomised controlled trials presented at ACC 2019, STOPDAPT-26 and SMART-CHOICE7, showed that one- or three-month DAPT followed by P2Y12 inhibitor monotherapy was superior for bleeding and non-inferior for the composite ischaemic endpoint to 12-month DAPT at one-year follow-up. Furthermore, the results of a large-scale RCT in a high-risk PCI population (the TWILIGHT study) are awaited8.
When compared with these studies, the ASET pilot study is unique in that aspirin is completely eliminated from the antiplatelet regimen immediately after the procedure.
Limitations
The findings of the present study need to be considered as hypothesis-generating due to the single-arm design without formal sample size calculation.
Conclusion
Favourable results of the present study would justify a subsequent investigational phase testing the same treatment strategy and proof of concept in patients with non-ST-elevation myocardial infarction and ST-elevation myocardial infarction.
|
Impact on daily practice The ASET pilot study challenges the concept of the additional synergistic effect of aspirin under monotherapy of a potent P2Y12 inhibitor (ticagrelor or prasugrel) immediately after DES implantation. The study results could provide a scientific basis to perform a larger randomised trial that could have the impact of changing clinical practice regarding the use of antiplatelet therapy after coronary stenting. |
Guest Editors
This paper was guest edited by Adnan Kastrati, MD; Deutsches Herzzentrum, Munich, Germany, and Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
This trial is supported by grants from Boston Scientific and Daiichi Sankyo.
Appendix. Study collaborators
Marie-Angèle Morel, BSc, Cardialysis Clinical Trials Management and Core Laboratories, Rotterdam, the Netherlands; Jan. J. Piek, MD, PhD, Department of Cardiology, Amsterdam University Medical Center, Amsterdam, the Netherlands; Joanna J. Wykrzykowska, MD, PhD, Department of Cardiology, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Conflict of interest statement
R. Cavalcante is an employee of Boston Scientific. J.J. Piek is a consultant for Philips/Volcano and is a member of the advisory board of Abbott Vascular. R. Modolo has received institutional research funding from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb/Sanofi, CSL Behring, Eli Lilly/Daiichi Sankyo, Medtronic, Novartis and OrbusNeich, and is a consultant to Boston Scientific, Abbott Vascular, Medscape, Siemens Medical Solutions, Regeneron Pharmaceuticals Inc. (no fees), Roivant Sciences Inc, and Sanofi. Y. Onuma is a member of the advisory board of Abbott Vascular. P.W. Serruys is a consultant to Volcano, and is a member of the advisory board of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor A. Kastrati has no conflicts of interest to declare. The Guest Editor A. Vahanian is a consultant for Edwards Lifesciences.

