
In 2007, when transcatheter aortic valve implantation (TAVI) was commercialised in Europe for the management of symptomatic severe aortic stenosis, patient selection was relatively straightforward: candidates were old, frail, with multiple comorbid illnesses, and had an elevated logistic EuroSCORE (>20%) or Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score (>10%). The inclusion of risk prediction models in this selection process was curious, since these scores were never developed to determine treatment allocation and, moreover, the risk prediction algorithm included only patients who underwent surgical intervention, with few patients who could be deemed typical TAVI candidates. Rather, these surgical scores were initially developed to benchmark an individual surgeon or institution’s results and inform patients and their families of the risk of a given procedure. Not surprisingly, these risk scores proved to have poor discriminatory power for predicting 30-day or one-year mortality, particularly with respect to transcatheter valve replacement where, irrespective of STS-PROM (0-10%), the 30-day mortality approximates 1-4%1. Recent updates to the EuroSCORE (II) and STS-PROM and dedicated TAVI risk scores, such as the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) model, have been developed and require validation2.
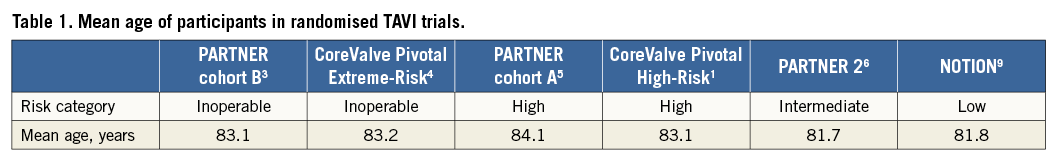
All early randomised trials comparing the safety and efficacy of TAVI to medical therapy in inoperable patients (Placement of Aortic Transcatheter Valve [PARTNER] trial cohort B3; CoreValve United States Pivotal Trial Extreme-Risk4) or surgical aortic valve replacement (SAVR) in high-risk patients (PARTNER trial cohort A5; CoreValve United States Pivotal Trial High-Risk1) included STS-PROM inclusion criteria cut-points. This selection strategy was deemed appropriate by physicians, regulators, payers, and guideline committees, as TAVI technology was unproven, and the extension of the technology to younger and fitter patients who could expect excellent surgical outcomes was undesirable. The aforementioned stakeholders artificially categorised patients with severe aortic stenosis using STS-PROM cut-points: low-risk <4%; intermediate-risk 4-8%; high-risk 8-15%; inoperable >15%.
More recently, TAVI has been compared to SAVR in patients of intermediate operative risk. Initially, the PARTNER 2 and SURTAVI trials determined intermediate risk based on clinical criteria and an STS-PROM cut-point (4-10%)6,7. In both studies, patient recruitment proved difficult due to the inflexibility of the STS-PROM score: a 98-year-old male with no comorbid illnesses undergoing isolated SAVR has an STS-PROM score of 3.8%, hardly a low-risk patient for either intervention! Protocol amendments subsequently removed the absolute necessity of the STS-PROM cut-points and established the supremacy of the institutional Heart Team in determining operative risk and suitability for inclusion. The Nordic Aortic Valve Intervention (NOTION) trial in contrast, a comparative effectiveness study of TAVI and SAVR in low-risk patients, was the first TAVI trial specifically to remove the STS-PROM from the decision-making algorithm8. These investigators included patients >70 years old who were deemed suitable for either surgical or transcatheter valve replacement.
It is interesting to note that randomised TAVI trials of excessive-, high-, intermediate-, and low-risk cohorts (adjudicated using STS-PROM) have failed to include younger patients: the mean age of participants remains above 80 years (Table 1). This is even true of the NOTION trial (mean age 81.8 years) which planned to enrol younger patients9. We can therefore be confident that TAVI works well in very elderly patients of varying frailty, but has the risk stratification employed to date enlightened our ability to determine the optimal treatment strategy for younger, non-octogenarian patients with severe aortic stenosis?
The role of the Heart Team, principally involving, but not limited to, the cardiac surgeon and interventional cardiologist, in determining the optimal treatment strategy for a given patient was initially conceived during patient screening for the Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) trial10. The application of this approach in cardiovascular medicine has however come of age in the selection of patients for transcatheter valve therapies. The effectiveness of the Heart Team in the realm of structural heart interventions is intuitive and, while the make-up and processes of individual Heart Teams may differ, a multidisciplinary team has the potential to improve patient selection and clinical outcomes, as demonstrated in the realm of cancer care. Further study confirming the Heart Team role in enhanced outcomes, cost reduction, and so forth is however required11.
In 2017 therefore, how should we select patients for TAVI? The answer to this question depends on the institution in question and, more importantly, on the outlook of the Heart Team at each institution. In many centres, very elderly and frail patients are no longer considered for SAVR and proceed directly to TAVI without discussion. Yet, in such instances the Heart Team can have an important role in determining the utility or futility of such cases. Similarly, young patients (<75 years) without comorbidity do not warrant discussion, as they should only have TAVI performed in the context of a clinical trial. In contrast, patients older than 75 years of age (high- or intermediate-risk) should have the benefit of a multidisciplinary Heart Team assessment of the clinical and anatomical complexities inherent to their particular case. Risk prediction scores can reinforce the decision of the Heart Team, are useful for clinical research, for benchmarking performance, and for helping patients and their families to understand the risks of the procedure. However, they should not be used to determine treatment allocation.
Conflict of interest statement
D. Mylotte and N. Piazza are consultants for Medtronic and MicroPort.

