Abstract
Aims: Delayed or incomplete stent endothelisation and stent malapposition may predispose to DES thrombosis that can be a catastrophic event in the left main coronary artery (LMCA). OCT can accurately identify stent struts and arterial tissue, but is limited by the need of vessel blood clearance and penetration; also no data exist on its use in LMCA. We sought to verify whether optical coherence tomography (OCT) can accurately assess arterial wall response after drug eluting stent (DES) implantation in the LMCA.
Methods and results: OCT images were obtained in 15 patients (mean age 70.7±8.0 years) six months after LMCA DES implantation. Acquisitions were performed without proximal balloon occlusion during iso-osmolar contrast injection through a 6 Fr guiding catheter without side holes at a speed of 2-3 mL/sec. Offline image analyses were performed to evaluate assessable stent area (all slices), strut coverage, apposition, and abnormal tissue responses for every three slices (= every 0.45 mm). All OCT images were obtained without periprocedural complications. Overall, 69±20% of the stent inner area was analysable; this corresponded to 2.7±0.8 analysable quadrants per slice. Out of 1,281 struts, 1,136 (88.7%) were well apposed and fully covered, 101 (7.8%) were uncovered; and 45 (3.5%) were malapposed although nine of the malapposed struts (20%) were covered by some tissue. In five patients OCT detected abnormal intraluminal tissue, and in two cases this finding was related to uncovered struts.
Conclusions: OCT assessment of vascular response after LMCA DES implantation is safe and feasible. Further development of OCT imaging technology will be necessary for complete evaluation of LMCA stents.
Introduction
After the clinical introduction of drug eluting stents (DES), percutaneous coronary intervention (PCI) is increasingly used to treat complex and high risk coronary lesions such as unprotected left main coronary artery (LMCA) disease1-6. Stent thrombosis (ST) is an uncommon, but dreadful complication of DES implantation manifesting as sudden death or acute myocardial infarction7,8. ST is a particularly catastrophic event when it occurs in the LMCA9. Delayed healing, poor endothelisation, and malapposition of stent struts are the most common pathological findings in late ST patients10.
Optical coherence tomography (OCT) is an intravascular imaging technique that provides high image resolution of the arterial wall (10-20 µm) and allows visualisation of stent struts. This technique has been used for in vivo assessment of arterial healing after stent implantation in animal models11 and in humans12,13. The applicability of this technique in LMCA has been considered to be limited because of the need of proximal occlusion and the issue of penetration. In fact, optimal OCT vessel assessment is confined to vessels <4 mm in diameter. To date, no study has evaluated the use of this imaging technique in the LMCA. Recently, a non-occlusive technique with infusion of iso-osmolar contrast agents has resulted in excellent feasibility, safety, and efficacy without the limitations and risk of LMCA proximal balloon occlusion11,13,14. In the present study we sought to evaluate whether non-occlusive OCT imaging can accurately assess arterial wall healing after stent implantation in the LMCA. This information might be useful to better stratify individual risk and to customise antiplatelet therapy.
Methods
Patient population
Between October 2008 and March 2009, 18 consecutive patients previously treated by unprotected LMCA DES implantation underwent scheduled 6-month follow-up angiography at our centre as recommended by PCI guidelines15. After conventional coronary angiography non-occlusive OCT images were obtained in 15 patients while, three patients were excluded because of severe left ventricular dysfunction (ejection fraction less than 20%), sub-occlusive LMCA in-stent restenosis requiring PCI, and no informed consent to perform OCT. The study was approved by the institutional ethics committee, and all 15 patients provided written informed consent.
OCT images acquisition
The LMCA was cannulated with a 6 Fr extra backup guiding catheter without side holes. To avoid the risk of masking the very proximal part of the LMCA, the guiding catheter was gently advanced, paying attention to approach, but without intubating the tip of the catheter into the ostium of LMCA. The OCT wire (Image-wire™, Westford, MA, USA) was then advanced distal to the LMCA bifurcation into the main daughter vessel – the left anterior descending artery (LAD, n=13) or the circumflex artery (LCX, n=2) according to the index procedure stent implantation technique. Given the goal of the current analysis (the study of the LMCA stent by OCT), we limited our OCT evaluation to the main vessel stent (the LMCA stent whether it crossed-over the LAD or LCX) and did not include the side branch stent or other stents in these patients. Iodixanol 320 (Visipaque™, GE Healt Care, Cork, Ireland), an iso-osmolar contrast agent, was injected manually via the guiding catheter at a speed of 2-3 mL/sec. during continuous electrocardiographic monitoring. We attempted to optimise the efficacy of flushing and to obtain a stable position of the guiding catheter during the contrast agent injection in all patients. The M3 OCT Imaging System (LightLab Imaging Inc., Westford, MA, USA) was used for the 20 frames/sec. OCT images acquisition during a 3 mm/sec. Image-wire pull-back speed. Image acquisition was repeated after five minutes. The acquisition run with the best image quality was used for the off-line analysis.
OCT images analysis
OCT images were analysed offline at an independent core laboratory (Cardiovascular Research Foundation, New York, NY, USA) separately by two experts in intravascular imaging (AM, TK). In case of disagreement a consensus was reached. OCT analysis has been shown to be highly reproducible14. We compared the reproducibility as intra-class correlations. One indicates perfect agreement and zero indicates no agreement. Generally >0.9 can be considered an excellent agreement and >0.8 is a very good agreement. Intra-class correlation across readers was 0.988 for fully analysable quadrants, 0.988 for strut count, and 0.965 for neointimal hyperplasia (NIH) thickness. The analysis focused on the segment of LMCA from the distal bifurcation (ostium of the LAD or of the LCX) to the LMCA ostium (or catheter tip). The analysis was performed for all slices to evaluate the analysable LMCA stent area, and at single strut level every 0.45 mm of stent sampling to evaluate analysable strut coverage, apposition, and abnormal tissue response. Each stent slice was subdivided into four quadrants: quadrant 1=1-90°, quadrant 2=90-180°, quadrant 3=180-270°, and quadrant 4=270-360°. The number of fully assessable quadrants per slice was calculated. The primary endpoint of the study was the analysable LMCA stent area. Secondary exploratory endpoints were the percents of covered, uncovered, or malapposed stent struts. A covered stent strut was defined when the distance between the centre of the reflection of the stent and the surface of tissue was more than 20 µm; otherwise, it was defined as an uncovered strut. NIH thickness was calculated as the distance between endoluminal surface and stent strut. A malapposed strut was classified when the distance between the centre of the reflection and the vessel wall was more than (thickness of stent strut +20 µm) (Figure 1).
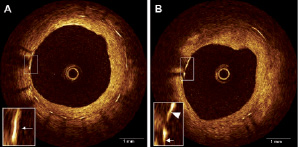
Figure 1. Heterogeneous left main coronary artery response six months after paclitaxel-eluting stent implantation assessed by non-occlusive OCT. Non-uniform stent strut coverage (A), and non-coverage (B; from 9 to 10 o’clock).
Abnormal intraluminal tissue (AIT) was defined as any tissue within the vessel wall surface, sub-categorised as (1) related to covered strut or non-covered strut, (2) with/without attenuation, and (3) irregular or smooth surface (Figure 2).
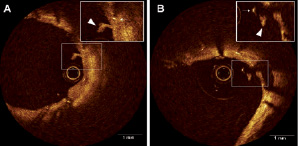
Figure 2. Abnormal intraluminal tissue (AIT). 2A showed AIT (triangle) with covered stent strut (arrow) which did not have attenuation. Figure 2B showed AIT (triangle) with non-covered stent strut (arrow)
Results
Study population and index procedure characteristics
Among the 15 enrolled patients the mean age was 70.7±8.0 years; a history of hypertension, diabetes mellitus, and dyslipidaemia was present in 60%, 26.6%, and 46.6%, respectively. All but two patients presented with involvement of the distal part of the LMCA. Provisional T-stenting technique was successfully adopted in 12 patients with the intent of maximal LMCA coverage. In only one patient two DES were implanted according to the crush technique. Eight patients received a paclitaxel eluting stent and seven an everolimus eluting stent in the LMCA. Mean LMCA stent diameter was 3.80±0.41 mm (ranging from 3.5 to 4.5 mm). Post procedure minimal luminal diameter was 3.77±0.25 (ranging from 3.5 to 4.3 mm). Quantitative coronary angiographic data and characteristics of the stents implanted in the LMCA are showed in Table 1.
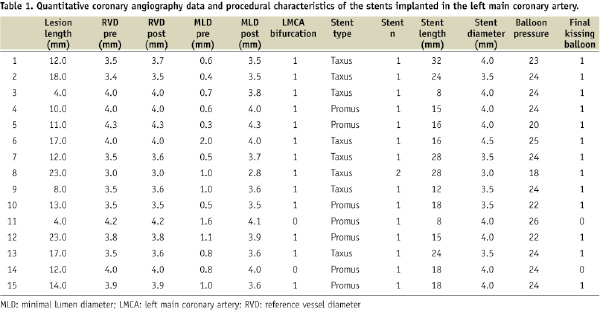
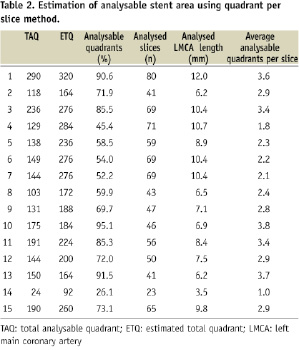
The stent length in Table 1 indicates total stent length covering the LMCA into the LAD or LCX. Our analysis was focused on the left main segment and included only the left main part of the stent; therefore, it was considered not correct to use the total stent length to “calculate” the analysed cross sections. Because the lumen diameter of the LMCA was usually bigger than 3 mm and the OCT Image-wire was often not in the centre of the lumen, many struts were out of view; therefore, we were not able to evaluate the whole lumen in all the slices in all the patients. Figure 2 clearly indicated such examples. Thus, if the lumen was too big and/or if the catheter was not located at the centre of the lumen, the number of analysable quadrants was low (see case No.14) and the number of analysable struts was very low (also see case No.14). On the other hand, in cases No.1, 10, and 13 (Table 2), because the catheter was located in the centre of the lumen, analysable quadrants were more than 90%. Because the primary endpoint of this study was to evaluate the «analysable LMCA stent area» and not the number of individual struts, we analysed all the slices in LMCA (Table 2). The detailed strut level analysis was only a secondary endpoint.
OCT safety
All OCT images were obtained successfully. The contrast agent injection displaced all the blood from the LMCA without the need for proximal balloon occlusion. The duration of pull-back ranged from six to 11 seconds. Mean pull-back time and OCT related contrast agent amount were, respectively, 8±3 seconds and 17±14 mL. During flushing, two patients described chest pain; and four patients had transient ST segment depression. No patient had arrhythmias, cardiac biomarker elevation, or contrast-induced nephropathy.
OCT feasibility
The length of the analysed coronary segments varied according to the implanted stent length (8.3±2.3 mm). A total of 829 slices were analysed (55.2±15.4 per patient) corresponding to 3,316 quadrants. Overall, 69±20% of the image slices had analysable stent areas; this corresponded to a mean of 2.7±0.8 fully analysable quadrants per slice (Table 2). At the strut level (Table 3), 1,281 struts could be assessed.
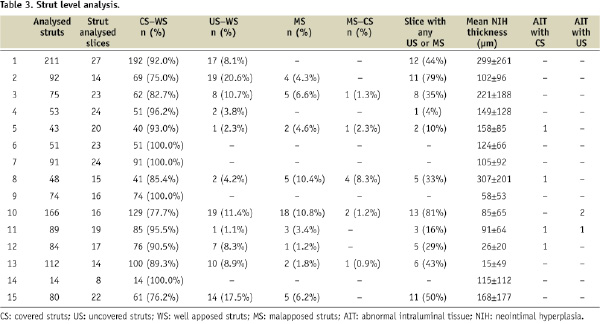
Overall, 1,136 struts (88.7%) were well apposed and fully covered; 101 struts (7.8%) were uncovered; and 45 struts (3.5%) were malapposed although nine of the malapposed struts (20%) were covered by some tissue. In our study, there was no significant difference in the percent of uncovered struts between patients with a paclitaxel eluting stent (n=8) as compared with those with an everolimus eluting stent (7.2±2.5% versus 6.4±2.4%; p=0.953). Five patients had AIT, three patients had AIT with covered struts, one patient had AIT with an uncovered-strut, and one patient had both. Out of four AITs with covered struts, half showed an irregular surface. Out of the three AITs with uncovered struts in two patients, two AITs showed an irregular surface. None of AITs had attenuation that would have indicated red-cell rich thrombus.
Discussion
The main finding of this study is that OCT assessment of vascular response after LMCA stenting is safe, feasible, and allows the analysis of an average of at least 2/3 of the previously implanted DES cross-sections.
OCT safety and feasibility
In the current study OCT images were obtained safely without procedure-related complications with only a limited amount of adjunctive contrast medium and time waste. OCT has been shown to be accurate in the identification and quantification of tissue coverage of stent struts13,14, a prerogative once only granted to pathologists evaluating tissues under the microscope. As in other coronary segments, OCT was able to identify LMCA stent struts that were covered (Figure 1A), uncovered, or malapposed (Figure 1B), as well as to accurately measure NIH thickness. All OCT coronary vessel examinations can be characterised by the proportion of the vessel that cannot be analysed due to limitations such as out-of-screen phenomenon, eccentric guidewire artefact, saw up artefact (rapid vessel motion beyond the temporal resolution of the system), bubbles artefacts, and residual blood artefacts16. In the case of LMCA that has an average diameter of 3.5-4.5 mm17, out-of-screen artefacts (Figure 2) were particularly relevant and limited the completeness of the evaluation of the DES implanted in the LMCA. Before the introduction of the non-occlusive OCT technique, it was not possible to evaluate proximal coronary segments such as LMCA. In our series the average of analysable quadrants per OCT slice varied widely and ranged from 26% to 95% with a mean value of 69±20%. This means that some stents were almost completely assessable, while other stents were very difficult to study. The variability depended on the heterogeneous impact of the aforementioned technical limitations and on LMCA diameter, length, take off, and curvature. LMCA diameter is probably the prevalent issue with the current OCT systems. Rapid movement of the vessel or of the Image-wire may contribute to reduced centricity of the OCT fibre during pullback as well as the centricity of the guiding catheter and the relationship between the parent vessel and the daughter vessel. Conversely, the lack of coaxiality between guiding catheter and the Image-wire may have influenced the centricity of the OCT Image-wire in relation to the vessel wall increasing the out-of-screen phenomenon in at least a limited number patients.
Clinical implications
Vessel healing after stenting occurs when the struts are covered by a layer of neointima and, eventually, endothelial cells. This tissue layer plays a pivotal role in preventing DES thrombosis, but it is usually so thin that it cannot be visualised by conventional angiography, IVUS, or TC angiography. Currently, OCT technique does not allow discrimination of cell types located in the strut-covering tissue. However, stent strut coverage has been associated with better outcome even in the absence of a more precise characterisation of covering tissue morphology18. Optimal evaluation of LMCA stent strut coverage may help to assess the risk of stent thrombosis and to guide clinical decision making on the duration of a tailored antiplatelet therapy for individual stent types or for individual patient. Likely, qualitative tissue analysis will improve with technology developments. Moreover, in the future OCT could be helpful for guidance of optimal stent implantation.
We acknowledge that the association between these findings at strut level and the clinical outcome has not been demonstrated. If in the future appropriately powered studies will demonstrate a potential link between OCT data and clinical events, OCT findings, as obtained in the present study, might be used as surrogate endpoints of clinical trials. Of note, the upcoming new frequency domain OCT technology (Fourier-Domain OCT system, Lightlab Imaging Inc., Westford, MA, USA) with larger scan diameter and higher acquisition speed (100 frame/s=20 mm/sec. pull-back speed) will at least in part overcome current limitations and will allow more complete evaluation of the LMCA.
Study limitations
First the present report suffers from its relatively limited study population. We recognise that this is a pilot exploratory study whose main objective was to assess the feasibility of LMCA stent evaluation by OCT; however, the data we provide may be of clinical relevance according to the current knowledge and available technologies. Second, by protocol, we evaluated the LMCA vascular response six months after DES implantation. Only a single time point evaluation has been performed, and we have no later follow-up invasive data. The optimal time point for stent struts analysis by OCT has still to be identified. Third, in at least a few of the cases with stent implanted at the true ostium of the LMCA, the very proximal struts could not be visualised due to the even minimal coronary engagement by the guiding catheter. Finally, concomitant IVUS assessment was not performed in these patients; thus, a head-to-head comparison for the assessment of OCT vs. IVUS imaging is not available. Of note, there is a paucity of published in formation about this issue although it is well known that OCT allows a more accurate assessment of the stent strut coverage, stent-vessel wall malapposition, thrombus-formation, plaque prolapse, and edge dissections due to the higher image resolution as compared with IVUS13,19,20. Whether these additional data will impact on long-term patient outcomes is currently not known.
Conclusions
OCT assessment of vascular response after LMCA DES implantation is safe and feasible. Further development of OCT imaging technology will be necessary for complete evaluation of LMCA stents.
Acknowledgements
This study was supported by an unrestricted grant from the Italian Health Ministry to the Tuscany Region for the Finalised Medical Research Program 2007.

