Abstract
Aims: Angiographic evidence of edge dissections has been associated with a risk of early stent thrombosis. Optical coherence tomography (OCT) is a high-resolution technology detecting a greater number of edge dissections –particularly non-flow-limiting– compared to angiography. Their natural history and clinical implications remain unclear. The objectives of the present study were to assess the morphology, healing response, and clinical outcomes of OCT-detected edge dissections using serial OCT imaging at baseline and at one year following drug-eluting stent (DES) implantation.
Methods and results: Edge dissections were defined as disruptions of the luminal surface in the 5 mm segments proximal and distal to the stent, and categorised as flaps, cavities, double-lumen dissections or fissures. Qualitative and quantitative OCT analyses were performed every 0.5 mm at baseline and one year, and clinical outcomes were assessed. Sixty-three lesions (57 patients) were studied with OCT at baseline and one-year follow-up. Twenty-two non-flow-limiting edge dissections in 21 lesions (20 patients) were identified by OCT; only two (9%) were angiographically visible. Flaps were found in 96% of cases. The median longitudinal dissection length was 2.9 mm (interquartile range [IQR] 1.6-4.2 mm), whereas the circumferential and axial extensions amounted to 1.2 mm (IQR: 0.9-1.7 mm) and 0.6 mm (IQR: 0.4-0.7 mm), respectively. Dissections extended into the media and adventitia in seven (33%) and four (20%) cases, respectively. Eighteen (82%) OCT-detected edge dissections were also evaluated with intravascular ultrasound which identified nine (50%) of these OCT-detected dissections. No stent thrombosis or target lesion revascularisation occurred up to one year. At follow-up, 20 (90%) edge dissections were completely healed on OCT. The two cases exhibiting persistent dissection had the longest flaps (2.81 mm and 2.42 mm) at baseline.
Conclusions: OCT-detected edge dissections which are angiographically silent in the majority of cases are not associated with acute stent thrombosis or restenosis up to one-year follow-up.
Introduction
Stent implantation is the preferred treatment for coronary artery stenosis. As a consequence of vessel trauma during stent implantation, edge dissections occasionally occur and may lead to abrupt or threatened vessel closure due to obstruction from prolapsing tissue or thrombosis. Previous reports have suggested an association between angiographic as well as intravascular ultrasound (IVUS)-detected edge dissections and early stent thrombosis (ST)1-5. At the same time, other studies evaluating the outcomes following stent implantation have shown that only a minority of patients with edge dissections experience clinical adverse events6-8. The impact of these findings and their management has therefore been debated.
Optical coherence tomography (OCT) is a high-resolution technology allowing a detailed assessment of the coronary vessel wall and implanted devices9. Consequently, OCT detects a higher number of edge dissections as compared to angiography and IVUS9,10. The often dramatic appearance of these vessel disruptions, even when angiographically silent, may generate concern for further complications and trigger additional stent implantation11,12. The question whether this is justifiable at a time when OCT is increasingly used during interventions has thus resurfaced. At present, the natural history and clinical implications of these findings in the short and long term remain insufficiently described. The objectives of the present study were to assess the morphology, healing response, and clinical outcomes of OCT-detected edge dissections using serial OCT imaging at baseline and at one year following drug-eluting stent (DES) implantation.
Methods
STUDY POPULATION
The study included serial data from the Copenhagen OCT registry and from the OCT substudy of the RESOLUTE all-comers trial13. The Copenhagen OCT registry was a single-centre prospective non-randomised evaluation of strut coverage and apposition at 12-month (average) follow-up in relation to apposition at baseline, using the following DES: CYPHER SELECT® Plus (Cordis, Johnson & Johnson, Warren, NJ, USA), TAXUS® Express™ (Boston Scientific, Natick, MA, USA), and Resolute® (Medtronic Inc., Santa Rosa, CA, USA). Patients were included between June 2008 and November 2010, and were eligible if they had ≥1 lesion with >50% diameter stenosis in a native coronary artery, with a reference vessel diameter between 2.25 and 4.0 mm. Exclusion criteria were ST-segment elevation myocardial infarction (MI), left ventricular ejection fraction <30%, renal insufficiency (creatinine >133 µmol/L), and lesion location in the left main stem or bypass graft. OCT as well as IVUS were performed after a satisfactory angiographic result, defined as a residual diameter stenosis <20% and Thrombolysis In Myocardial Infarction (TIMI) flow grade 3. It was prespecified that angiographically visible edge dissections occurring during the procedure would only be treated in case they were obstructive and flow-limiting (defined as dissections at least type C with a residual diameter stenosis >50% and a TIMI flow ≤2). Specifically, treatment of edge dissections visible by OCT was not permitted unless they fulfilled the angiographic criteria. Administration of glycoprotein (GP) IIb/IIIa inhibitors was left to the discretion of the operator. Whether this was a consequence of the dissection or not was noted. Imaging with both OCT and IVUS was repeated at 12-month (average) follow-up. Of note, six patients (out of a total 49 patients) imaged at baseline in the Copenhagen cohort were excluded due to withdrawal of consent for follow-up.
The RESOLUTE trial was a randomised multicentre all-comers non-inferiority trial comparing the Resolute and XIENCE V® (Abbott Vascular, Santa Rosa, CA, USA) stents14. Inclusion criteria were broad, including patients with chronic stable angina as well as acute coronary syndromes, having ≥1 lesion with >50% diameter stenosis with a reference vessel diameter between 2.25 and 4.0 mm. Exclusion criteria were known intolerance to any of the stent components, and planned surgery within six months of the index procedure. Administration of GP IIb/IIIa inhibitors was left to the discretion of the operator. The OCT substudy included patients randomly selected for angiographic follow-up at 13 months from centres where OCT was available. The principal endpoint was strut coverage at follow-up13. A limited number of patients, included between May and November 2008, also had a baseline evaluation. There were no per protocol prespecified instructions regarding the management of edge dissections; however, for the purpose of this study, the occurrence of angiographic edge dissections after stent implantation was noted and, if present, also whether these were treated with additional stent implantation.
From the serial data available, we included lesions exhibiting edge dissections as assessed by OCT after stent implantation. Both studies were approved by the local ethics committees and written informed consent was obtained from all patients prior to enrolment.
OCT AND IVUS ACQUISITIONS
OCT acquisitions were performed with commercially available systems (time-domain M2 and M3 systems; frequency-domain C7 system) from LightLab/St. Jude (Westford, MA, USA). OCT images were acquired at a frame rate of 15.6, 20, and 100 frames/s at a pullback speed of 1, 3 and 10 mm/s with the M2, M3 and C7, respectively. Acquisition with occlusive (M2) and non-occlusive (M3 and C7) techniques has been described previously15. IVUS images were acquired with the Atlantis SR Pro 40 MHz catheter and iLab system (Boston Scientific, Natick, MA, USA) at a frame rate of 30 frames/s and pullback speed of 0.5 mm/s, according to accepted standards16.
ANGIOGRAPHIC ANALYSIS
All angiograms were assessed by three independent cardiologists with regard to the presence of edge dissections. In case of disagreement, a consensus diagnosis was obtained. If present, the dissection was graded according to the National Heart, Lung, and Blood Institute classification17.
OCT IMAGE ANALYSIS
OCT pullbacks were analysed off-line using proprietary software from LightLab/St. Jude. The region of interest included reference vessel segments (RVS) 5 mm proximal and distal to the stent, which were analysed systematically at 0.5 mm intervals. Edge dissections were defined as disruptions in the luminal vessel surface in the RVS, with or without involvement of the stented segment.
Dissections were classified as flaps, cavities, double-lumen dissections or fissures (Figure 1A-Figure 1D), and their longitudinal extensions along the stented segment were measured. For flaps, the flap root thickness was assessed semi-automatically from the joint point with the vessel wall to the luminal surface along a line projected through the gravitational centre of the lumen. The flap length was measured from the joint point with the vessel wall to the tip of the flap. The flap area was measured as the area bounded by the flap root thickness trace and the luminal contours of the flap. For cavities, the cavity depth was measured along a line projected through the gravitational centre of the lumen, from the deepest point in the cavity to a virtual line extrapolated between the luminal vessel contours on each side of the cavity. The cavity width was quantified at its widest point as parallel to the virtual line as possible, and the cavity area was assessed as the area bounded by the luminal contour of the cavity and the help line extrapolated between the luminal vessel contour on each side of the cavity. Double-lumen dissections were those having a false lumen separated from the true lumen by a cap. The cap thickness was quantified semi-automatically from the joint point with the vessel wall to the luminal vessel contour along a line projected through the gravitational centre of the lumen, and the largest of the two cap thicknesses was used. The cap length was measured as the distance between the two joint points connected by a straight line, while the cap area was defined as the area bounded luminally by the vessel surface to the sides by the cap thickness traces, and in the depth by the false lumen. Fissures were present when a split was visible delineating a flap-like structure not lifted from the vessel wall, which otherwise displayed an uninterrupted luminal contour. Due to a poor demarcation of the root of the flap-like structure, no measurements were performed on the fissures at the cross-sectional level; however, they were included in the assessment of longitudinal extension as it was hypothesised that they were consistent with injury. The circumferential extension of the dissection was expressed as the number of quadrants involved. The axial injury of the dissection was described as intimal involvement when only the intima was affected and media was still intact, as medial involvement when the dissection extended into the media without disruption of the entire medial layer, and as adventitial involvement when the media was dissected throughout its thickness (Figure 1E-Figure 1G). In case the media was not discernible, the dissection was classified as involving only the intima. The lumen area was assessed in each selected frame of the RVS as the area bounded by the luminal vessel contour. The kappa value for inter-observer reproducibility of the classification of edge dissections at the lesion level was 1.0.
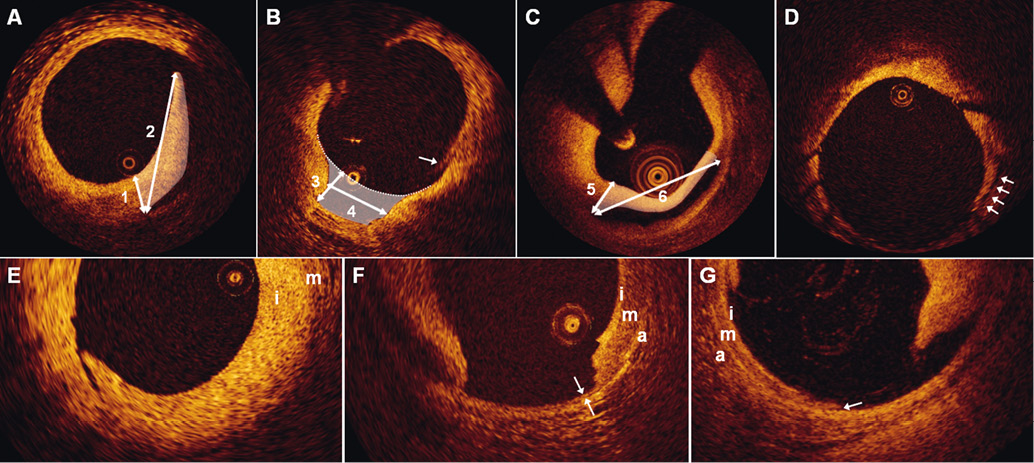
Figure 1. Classification of edge dissections. A) shows a dissection flap with indication of the flap root thickness (1), flap length (2), and flap area (white shading). B) shows a dissection cavity with the cavity depth (3), cavity width (4) and cavity area (white shading) displayed. The white arrow points to an artefact caused by non-uniform rotational distortion, and should not be misinterpreted as a dissection. The diagnosis was made by inspecting adjacent frames (not shown). C) shows a double-lumen dissection with indication of the largest cap thickness (5), cap width (6), and cap area (white shading). D) the arrows point to a fissure. Panels E-G show dissections involving only the intima (i) (E), the media (m) (F), and the adventitia (a) (G). F) the double arrows demarcate the only intact portion of the disrupted media. G) the arrow indicates the point where the media is lifted from the adventitia.
We also assessed the underlying tissue at the site of the dissections (at the lesion level) with respect to plaque type, which was classified as fibrous (>500 µm thick in at least one quadrant), fibrocalcific, or thin-cap (TCFA) fibroatheroma according to the international consensus (the two latter plaque types considered to be present when the calcified or lipid regions extended >1 quadrant)18. We also noted whether the extension was eccentric or concentric.
IVUS IMAGE ANALYSIS
IVUS pullbacks were analysed off-line using the QCU-CMS software (Medis, Leiden, The Netherlands) at standard 1 mm intervals in the described region of interest. Dissections were defined as tears in the intima-media with visualisation of blood speckle behind a flap or within a cavity or double lumen. The presence of dissections was assessed at the lesion level. The lumen area was measured in the RVS as the area bounded by the luminal vessel contour, whereas the external elastic membrane (EEM) area encompassed the area bounded by the interface between the intima-media layer and the adventitia. The plaque and media area was calculated as the EEM area minus the lumen area. The kappa value for inter-observer reproducibility of the presence or not of edge dissection by IVUS was 1.0.
CLINICAL OUTCOMES
Clinical outcomes in terms of death, MI, target lesion revascularisation (TLR) and target vessel revascularisation (TVR) were assessed for the Copenhagen OCT registry by two independent observers blinded to the imaging results, and for the RESOLUTE trial by a clinical events adjudication committee. The overall rate of stent thrombosis at one year was assessed according to the Academic Research Consortium definitions19.
STATISTICAL ANALYSIS
Continuous data were presented as means±standard deviations or median and interquartile ranges (IQR), depending on their distribution, which was assessed using the Kolmogorov-Smirnov test. Categorical data were presented as frequencies and percentages. The morphometric data at baseline and follow-up were compared using a paired t-test, and a two-sided p≤0.05 was considered statistically significant.
Results
CLINICAL AND PROCEDURAL CHARACTERISTICS
Out of a total of 57 patients with 63 lesions, 20 patients with 21 lesions and 22 OCT-defined edge dissections were included in the final analysis (Figure 2). Table 1 shows the baseline demographics. The average age was 63 years, 15 (75%) patients were males, and 14 (70%) patients presented with acute coronary syndrome. All lesions had a final TIMI 3 flow. Out of the 22 OCT-defined edge dissections, two (9%) were angiographically visible as type A haziness, and both were located within the same vessel (distal lesion with proximal dissection and proximal lesion with distal dissection), separated by only 2 mm. Of note, none of the 63 lesions was treated with additional stents, whereas five (25%) of 20 patients received treatment with GP IIb/IIIa inhibitors directly as a consequence of the OCT findings.
Results of OCT analysis
Out of the 62 assessable lesions with 58 distal and 30 proximal visible edges, 21 (34%) lesions with 16 (28%) distal and six (30%) proximal edges showed dissections, respectively. One of these lesions had both a proximal and distal dissection. Table 2 shows the characteristics of these dissections at baseline. Six (27%) dissections also involved the stented segments. None of the six patients from the Copenhagen cohort who withdrew consent for follow-up exhibited dissections at baseline examination.
Twenty (90%) out of 22 dissections were completely healed at follow-up (Figure 3). The two dissections that did not heal (Figure 4) exhibited the longest circumferential extension (2.81 and 2.42 mm), and in one case the longest longitudinal extension (6.0 mm) at baseline, and both were combinations of flaps and double-lumen dissections.
The morphometric results are presented in Table 3. Luminal dimensions in the reference vessel segment remained stable at follow-up.
The plaque type at the site of dissection was: an eccentric fibrous plaque in 17 (77.3%) lesions; concentric fibrous plaque in one (4.5%) lesion; eccentric fibrocalcific plaque in four (18.2%) lesions; and an eccentric TCFA in one (4.5%) lesion. In all cases of eccentric plaque, the point of dissection was at the transition site between the normal intima/thinnest point of plaque and the point where the plaque increases in thickness (Figure 2 and Figure 3). The two cases exhibiting signs of incomplete healing at follow-up had both underlying eccentric fibrous plaques.
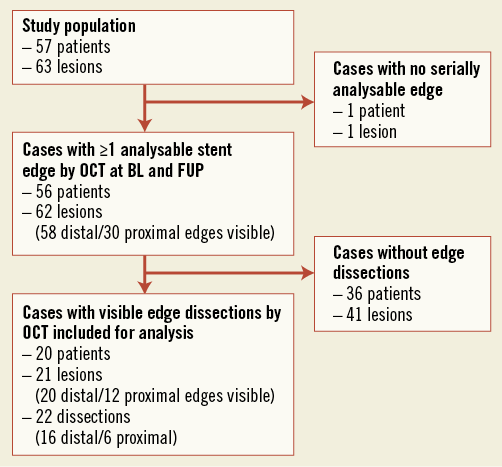
Figure 2. Flow chart.
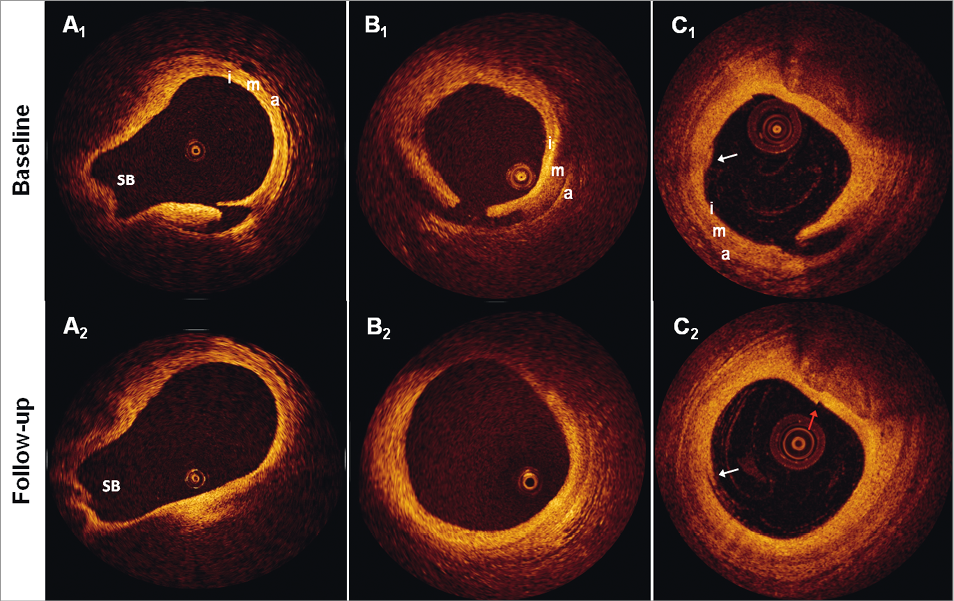
Figure 3. Examples of healed dissections. The figure shows three dissection sites (A-C) at baseline and follow-up. Of note, there were no changes in signal intensity from baseline to follow-up at any of the dissection sites in the study. SB: side branch; i: intima; m: media; a: adventitia; white arrows in C1 and C2 point to residual blood in the vessel lumen, which should not be mistaken for dissections (diagnosis made by inspecting adjacent frames (not shown)); red arrow in C2 points to a seam line artefact, which should not be mistaken for a dissection.
RESULTS OF IVUS ANALYSIS
IVUS imaging at baseline was available for 18 (82%) lesions, of which nine (50%) exhibited edge dissections. The results of serial morphometric analyses from 17 (77%) lesions are presented in Table 3. The lumen and EEM areas of the RVS increased slightly at follow-up; however, the plaque and media area remained stable.
CLINICAL OUTCOMES
All patients but one received dual antiplatelet therapy with aspirin and clopidogrel during the entire one-year follow-up period. One patient received clopidogrel alone. There were no deaths, MIs, TLRs or stent thromboses during the entire follow-up for any of the patients. However, at the one-year imaging procedure one patient had a clinically driven TVR. The two patients with lesions exhibiting signs of persistent edge dissections at one-year follow-up were free of any major adverse cardiac events up to three years after the index procedure. None of the six patients from the Copenhagen cohort who had withdrawn consent for a follow-up OCT examination experienced any events at follow-up.
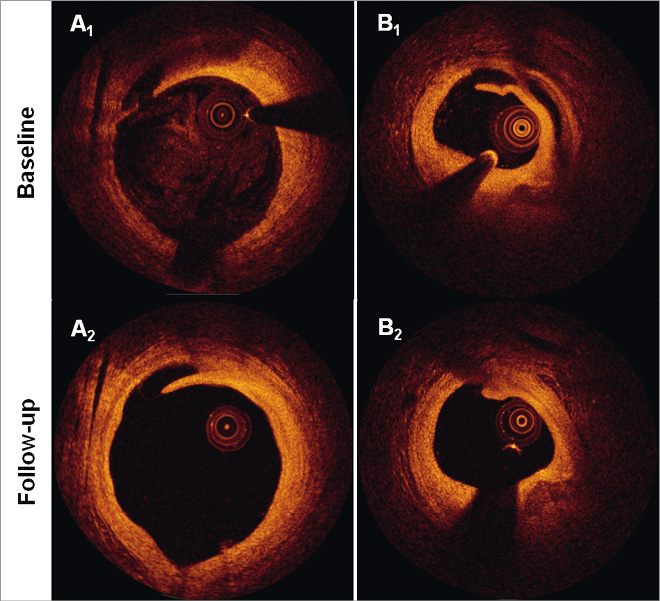
Figure 4. Incompletely healed dissections. The figure shows the serial appearance of the two incompletely healed edge dissections (A-B). Of note is that the flap length in both cases was reduced at follow-up, although the dissection in panel A extended slightly longer longitudinally.
Discussion
Percutaneous coronary interventions are inevitably associated with vascular injury, including intimal tears and medial disruption20,21. Using a systematic classification and quantification of the extent of injury in three dimensions, we studied the natural history of OCT-detected non-flow-limiting edge dissections and found the following: 1) edge dissections were in principle constituted by dissection flaps, although cavity, double lumen and fissure formation were also seen; 2) the majority of OCT-detected edge dissections healed uneventfully without excessive tissue formation; and 3) OCT-detected edge dissections, most of which were angiographically silent, were in this series of patients associated with favourable procedural outcomes and midterm prognosis.
INCIDENCE OF EDGE DISSECTIONS AND MECHANISM OF HEALING
The incidence of edge dissections following stent implantation ranges from 1.7-6.4%1,2 by angiography, increasing to 7.8-19.3% by IVUS7,8,22, and 26.3%10 up to 34% seen by OCT in the present study. This not only reflects differences in resolution, but also indicates that the use of intracoronary imaging tools more often requires a decision regarding management of apparently “imperfect” results. Published evidence, as well as the notable (25%) dissection-triggered use of GP IIb/IIIa inhibitors in the present study, confirm that OCT-detected edge dissections may cause concern for further events11,12, although their sequelae up until now have not been sufficiently explored. Our data show that the majority of edge dissections healed completely during follow-up with only two out of 22 lesions showing residual signs of injury at one year. As indirectly suggested by the absence of an increase in plaque area by IVUS, the mechanism of healing was previously proposed to be a “layering” or “tacking down” rather than filling in of the gap with plaque material7 –something which OCT in our study provided direct evidence for. It may be hypothesised that this is the consequence of the normal physiological response to injury, where fibrin deposition at the disrupted surfaces of exposed tissues contributes to “gluing” the dissected layers together following the approximation of fluttering tissue flaps against the vessel wall by the flowing blood. It therefore seems reasonable to assume that large flaps, in particular in the circumferential direction, are more mobile than smaller ones, and may not only increase the risk of obstruction and thrombosis, but also predispose to delayed vessel healing if continuously being separated from the vessel wall by blood, somewhat analogous to observations from wound healing23. In fact, the two edge dissections which healed incompletely at follow-up in the present study exhibited the largest flap length (2.81 and 2.42 mm) at baseline and thus the longest circumferential extension in the entire cohort. Due to the low incidence of incomplete healing, we can only speculate that the maximum flap length may be more important for predicting healing than the extension in the axial and longitudinal direction, since the former is perpendicular to the blood flow. However, the combination with a double-lumen formation, kept open by the blood stream, may also play a role and could possibly have been the cause of the slight progression in longitudinal length in one of these cases at follow-up (from 1.8-2.0 mm). Although the sample size was relatively low, as were the frequencies of TCFA and fibrocalcific plaques at the stent edges, there seemed to be no particular association between the underlying plaque type and propensity of healing.
In terms of healing of edge dissections, our data are in line with a recent report by Kume et al24 who observed, in a total of 36 patients (39 lesions) with 12 edge dissections, that all but one were fully healed at follow-up. However, the following differences should be noted: data in the present study were collected prospectively (vs. retrospectively); with prespecified instructions on the interventional strategy in 45/63 (71%) lesions (vs. strategy left to the discretion of the operator); with similar follow-up time in all patients (vs. median (range) follow-up of 188 (98-461) days); solely with the use of DES (vs. DES+bare metal stents [BMS]); with a methodology comprising all types of edge dissections including cavities and double-lumen dissections which occurred in 13.6% to 36.4% of cases (vs. flaps alone). Although the number of non-healed dissections at one year was very low, the present study provides details on the characteristics that may have contributed to the lack of complete healing, and which may be regarded as hypothesis-generating for the further understanding of factors of importance for vessel healing.
CLINICAL IMPORTANCE OF EDGE DISSECTIONS
The absence of early events in our study is in line with previous IVUS reports and the OCT report by Kume et al, indicating that there appears to be no increased risk of early stent thrombosis in lesions with residual edge dissections as compared to those without8,24-26. In the present study, this may be attributed to several factors: 1) the small injury defect; 2) an early “tacking down” limiting the risk of obstruction and thrombosis; and 3) a potent antithrombotic and antiplatelet therapy. However, the fact that the vast majority of dissections were angiographically silent and half were unidentified by IVUS indicates that they were relatively smaller in comparison to those previously associated with early stent thrombosis, including angiographic type B-F dissections1-3. Most importantly, however, these and other studies unanimously showed that, in addition to edge dissections, other “abnormal findings” were concurrently present by angiography and IVUS, most consistently a low final minimal lumen diameter/area and poor stent expansion, but also a reduced final TIMI flow <34,5,27,28, suggesting that stent edge dissections may not per se cause acute events.
EDGE DISSECTIONS AND RESTENOSIS
In the long term, our morphometric analysis showed an absence of restenosis at the site of previous dissection despite the presence in several cases of deep vessel injury involving the media and/or adventitia, something otherwise known to be associated with a hyperproliferative response29. Our results confirm previous findings by Sheris et al who similarly observed that the plaque and media area in the RVS by IVUS remained stable over a period of six months, even in the presence of submedial dissections7. It could be speculated that the antiproliferative drug from the DES in our study could have had a beneficial effect in preventing restenosis even in the RVS. However, considering that Sheris et al found the same in patients with BMS, together with other data showing that non-occlusive type A-D dissections following balloon angioplasty were associated with a reduced risk of restenosis compared to lesions without dissections6, it is likely that the process of “tacking down” by the flowing blood was more important, as it is well known that wounds with separated edges often heal with extensive scar formation, compared with wounds with approximated edges which heal with hairline scars23.
As it relates to the differences in lumen areas between the various imaging techniques used, it is well known that areas as measured by IVUS tend to be larger than by OCT30-32 (with the old as well as the new OCT systems), and our measurements are fully in line with this. The differences have occasionally been shown to be very large (25-72% difference)31. This can be caused by the use of an occlusion balloon; however, another possible cause is the relatively greater degree of cardiac motion by IVUS due to a very slow pullback (0.5 mm/s) as compared to OCT (1-20 mm/s), resulting in a relatively larger “imprecise” matching between baseline and follow-up. In addition, with the resolution of OCT being 10-20 µm, and that of IVUS 100-150 µm, “inaccuracies” within the resolution window are relatively larger by IVUS (±100-150 µm) than by OCT (±10-20 µm) which may result in significant differences32.
MANAGEMENT OF OCT-DETECTED RESIDUAL EDGE DISSECTIONS
Due to the absence of adverse events in the present cohort, we cannot provide any quantitative directives on which dissections will cause events and which will not. However, longitudinal extensions up to an average of 7.8 mm in previous IVUS studies25, and a circumferential extension up to 2.81 mm by OCT as seen in our study, were both associated with an uneventful midterm clinical course. The additional mechanical treatment of OCT-detected residual edge dissections being mainly angiographically silent should therefore be carefully evaluated, not least when considering that additional IVUS-guided stent implantation for residual edge dissections has not been shown to entail any benefits in terms of reducing the rate of restenosis and stent thrombosis when compared to an angiographically guided group without treatment of these injuries24, but was rather associated with complications in terms of TLR8. Considering that GP IIb/IIIa inhibitors seem to exert a protective effect against early stent thrombosis3,33, these may be a reasonable alternative to stent implantation in the presence of large non-flow-limiting OCT-detected edge dissections.
Limitations
The number of patients in this observational study was relatively small, mainly related to the requirement of serial assessment with OCT and IVUS. Furthermore, the inability of OCT to image proximally/ostially located regions, due to the need for blood clearance during acquisition, precluded visualisation of all proximal reference vessel segments. Together with a certain selection of patients, the dissection incidence of 34% may not reflect that in the real world, although it may be assumed that they are fairly representative for OCT-detected edge dissections which are for the most part angiographically silent. The low number of patients further limited our ability to detect rare adverse events and thus to provide recommendations on when a residual dissection imposes an increased risk of, e.g., acute stent thrombosis. Finally, we did not assess the predictors of edge dissections as our focus was on their natural history, and as predictors had previously been evaluated8. Furthermore, considering that the majority of these dissections underwent complete healing, this may seem redundant.
Conclusion
OCT-detected edge dissections, which are angiographically silent in the majority of cases, constitute a relatively frequent and benign finding after DES implantation, and are not associated with acute stent thrombosis or restenosis up to one-year follow-up.
Guest Editor
This paper was Guest Edited by Michael Joner, MD; Facharzt für Innere Medizin und Kardiologie, Deutsches Herzzentrum München, Technische Universität, Munich, Germany.
Acknowledgements
M. Radu has received research grants from The Heart Centre Rigshospitalet Research Foundation and Copenhagen University. L. Räber is the recipient of a research fellowship (SPUM) funded by the Swiss National Science Foundation (Grant 33CM30-124112).
Conflict of interest statement
S. Windecker has received consulting and lecture fees from Abbott, Boston Scientific, Biosensors, Cordis, and Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

