Abstract
BACKGROUND: Little is known about the occurrence of subclinical new-onset atrial fibrillation (NOAF) after transcatheter aortic valve implantation (TAVI).
AIMS: We aimed to evaluate the incidence, predictors, and clinical impact of subclinical NOAF after TAVI.
METHODS: This was a multicentre study, including patients with aortic stenosis (AS) and no previous atrial fibrillation undergoing TAVI, with continuous ambulatory electrocardiogram (AECG) monitoring after TAVI.
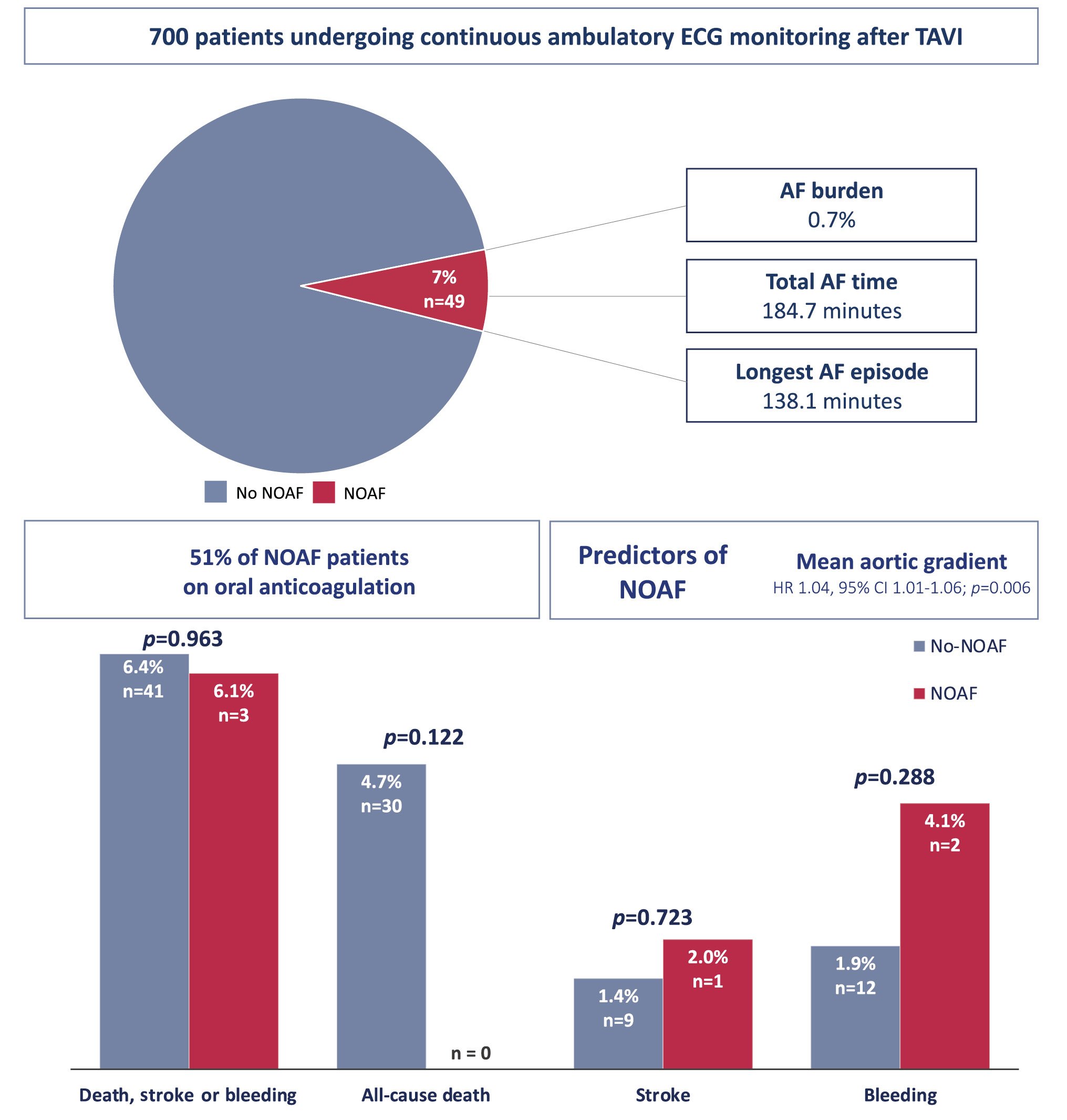
RESULTS: A total of 700 patients (79±8 years, 49% female, Society of Thoracic Surgeons score 2.9% [1.9-4.0]) undergoing transarterial TAVI were included (85% balloon-expandable valves). AECG was started 1 (0-1) day after TAVI (monitoring time: 14121314 days). NOAF was detected in 49 patients (7%), with a median duration of 185 (43-421) minutes (atrial fibrillation burden of 0.7% [0.3-2.8]). Anticoagulation was started in 25 NOAF patients (51%). No differences were found in baseline or procedural characteristics, except for a higher AS severity in the NOAF group (peak gradient: no NOAF: 71.9±23.5 mmHg vs NOAF: 85.2±23.8 mmHg; p=0.024; mean gradient: no NOAF: 44.4±14.7 mmHg vs NOAF: 53.8±16.8 mmHg; p=0.004). In the multivariable analysis, the baseline mean transaortic gradient was associated with a higher risk of NOAF after TAVI (odds ratio 1.04, 95% confidence interval: 1.01-1.06 for each mmHg; p=0.006). There were no differences between groups in all-cause mortality (no NOAF: 4.7% vs NOAF: 0%; p=0.122), stroke (no NOAF: 1.4% vs NOAF: 2.0%; p=0.723), or bleeding (no NOAF: 1.9% vs NOAF: 4.1%; p=0.288) from the 30-day to 1-year follow-up.
CONCLUSIONS: NOAF detected with AECG occurred in 7% of TAVI recipients and was associated with a higher AS severity. NOAF detection determined the start of anticoagulation therapy in about half of the patients, and it was not associated with an increased risk of clinical events at 1-year follow-up.
Transcatheter aortic valve implantation (TAVI) has become a well-established alternative for treating severe symptomatic aortic stenosis (AS), regardless of surgical risk1. New-onset atrial fibrillation (NOAF) incidence in patients undergoing TAVI is around 7-10%23. However, in patients with a pacemaker or an implantable loop recorder, the prevalence of NOAF rises to 25% or even to 82%456.
Atrial fibrillation (AF) in TAVI patients is related to poorer outcomes, with a higher risk for mortality and cerebroÂvascular events378. For those patients with NOAF after TAVI, the increased risk of stroke persisted up to the 2-year follow-up, which might reflect the heterogeneous management of anticoagulation in the setting of periprocedural AF3.
The electrocardiogram (ECG) is the most commonly employed method for arrhythmia detection after TAVI3. Continuous ambulatory ECG (AECG) monitoring improves the diagnosis of subclinical arrhythmic episodes in high-risk patients9. Thus, AECG after TAVI, used mostly to detect conduction disturbances, could also unmask subclinical tachyarrhythmic episodes in this particularly comorbid population56. However, to date, there is no available evidence on the associated factors and prognostic implications of subclinical AF episodes that could help in the decision-making process for selecting the most appropriate therapeutic approach3. This study aimed to evaluate the incidence, predictors, and clinical impact of subclinical NOAF after TAVI.
Methods
STUDY POPULATION
This was a multinational, multicentric, observational analysis of prospectively collected data, including patients who had undergone a TAVI procedure in one of three university centres in Canada and the USA between 2014 and 2022. Patients who underwent AECG after the procedure were selected for inclusion in the study. Patients underwent AECG as part of previous prospective observational research projects101112 or based on clinical indications dependent on each centre’s local protocol (Supplementary Figure 1). The MARE Study screened 1,584 consecutive TAVI patients (June 2014-July 2016). After excluding patients who did not fulfil the inclusion criteria for the study, it finally recruited 103 patients with persistent left bundle branch block after TAVI10. The RECORD Study11 screened 750 consecutive patients undergoing TAVI between July 2019 and November 2020 and finally included 459 patients. The REdireCT TAVI study12 evaluated 672 consecutive patients referred to the valve clinic between June 2018 and March 2020 and finally included 192 patients who underwent AECG after TAVI. Finally, in 2021 and 2022, among 1,435 consecutive patients undergoing TAVI, 258 patients underwent AECG based on clinical indications (i.e., persistent left bundle branch block, right bundle branch block, increased PR or QRS interval, transitory heart block during the procedure).
A total of 1,012 TAVI patients with post-TAVI AECG were screened for their inclusion in this analysis. Patients with a previous diagnosis of AF (287 [28%]) and those who developed NOAF during the index hospitalisation (20 [2%]) were not included in this analysis. Five patients (0.5%) undergoing non-transarterial access were also excluded.
Each local Heart Team assessed the indications for TAVI, device type, and procedural approach based on an extensive clinical and anatomical preoperative assessment. The transfemoral approach was preferred, and an alternative transÂarterial (transcarotid, transsubclavian, or transaxillary) access was used for patients with unfavourable iliofemoral anatomy. This study was conducted according to the ethics committee of each participating centre, and all patients provided signed informed consent before their inclusion.
ECG MONITORING AND ARRHYTHMIC EVENTS DEFINITION
Patients received an AECG device (CardioSTAT [Icentia]: 204 patients, 29%; Zio AT [iRhythm Technologies]: 274 patients, 39%; Reveal LINQ [Medtronic]: 76 patients, 11%; or Pocket ECG device [m-Health Solutions]: 146 patients, 21%) before hospital discharge. In patients monitored with an implantable loop recorder (Reveal LINQ), the monitoring time for the detection of NOAF was restricted to the 30 days following the implant. All types of arrhythmic events were automatically recorded. AECG tracing was adjudicated by a cardiologist to confirm the occurrence of arrhythmic events. AF was defined as the presence of an atrial rhythm with no discernible P waves and irregular RR intervals lasting ≥30 seconds13. The time of monitoring, the total time in AF, and the duration of the longest AF episode were recorded. AF burden was defined as the percentage of the total monitored time the patient was in AF.
DATA COLLECTION AND STUDY OUTCOMES
Baseline, procedural, and follow-up data were prospectively collected in each dedicated local TAVI database. The patient’s pre-TAVI workup and follow-up were performed according to local protocols. The need for additional diagnostic tests or treatment changes was left to the discretion of each patient’s physician, with no specific recommendations regarding the need for oral anticoagulation (OAC) or any other medical treatment. The patient’s vital status was updated after every medical contact, and the date of the last contact was recorded for every patient. For this non-prespecified analysis, we collected information about mortality and the occurrence of stroke and bleeding events.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation or median (first quartile [Q1]-third quartile [Q3]) according to the normality of data distribution assessed with the Shapiro-Wilk test. Categorical variables are expressed as frequency (%). The chi-square or Fisher’s exact test were used to compare the categorical variables. The Student’s t-test or the Mann-Whitney U test were used to compare continuous variables. Factors associated with the development of NOAF were determined using logistic regression analysis. Variables with p-values<0.10 in the univariable analysis were included in the multivariable model.
For the 1-year survival analysis, the Kaplan-Meier method was used. The difference between the probability of event occurrence was assessed with the log-rank test. A Cox proportional hazards regression analysis was performed to evaluate the impact of NOAF detected during the AECG continuous monitoring. A two-sided p-value<0.05 was considered significant for all statistical tests. All data were analysed using the statistical package Stata, version 15.0 (StataCorp).
Results
A total of 700 patients (78.8±7.9 years, 49% female, median Society of Thoracic Surgeons score 2.9 [1.4-4.0]%) were included in the analysis (Table 1).
Table 1. Patients’ baseline and procedure-related characteristics.
| Total N=700 | No NOAF N=651 | NOAF N=49 | p-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 78.8±7.9 | 78.6±7.9 | 80.7±8.5 | 0.073 |
| Female | 342 (49) | 316 (49) | 26 (53) | 0.542 |
| Body mass index, kg/m2 | 29.0±6.5 | 29.0±6.5 | 29.2±6.9 | 0.881 |
| Hypertension | 611 (87) | 565 (87) | 46 (94) | 0.151 |
| Diabetes mellitus | 247 (35) | 232 (36) | 15 (31) | 0.478 |
| Coronary artery disease | 355 (51) | 328 (50) | 27 (55) | 0.531 |
| Chronic kidney disease | 165 (24) | 152 (24) | 13 (27) | 0.669 |
| Chronic pulmonary artery disease | 108 (15) | 101 (16) | 7 (14) | 0.815 |
| Previous stroke | 73 (11) | 68 (11) | 5 (10) | 0.975 |
| Peripheral artery disease | 82 (12) | 73 (11) | 9 (18) | 0.133 |
| CHA2DS2-VASc score | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | 0.383 |
| STS score, % | 2.9 (1.9-4.0) | 2.9 (1.9-4.0) | 3.2 (2.2-3.8) | 0.419 |
| Indexed left atrial volume, mL/m2 | 39.2±12.0 | 39.0±12.0 | 42.1±11.9 | 0.362 |
| Left ventricular ejection fraction, % | 57.8±10.1 | 57.8±10.1 | 58.5±9.7 | 0.649 |
| Aortic valve area, cm2 | 0.78±0.26 | 0.78±0.25 | 0.78±0.41 | 0.972 |
| Aortic gradient – peak, mmHg | 72.6±23.6 | 71.9±23.5 | 85.2±23.8 | 0.024 |
| Aortic gradient – mean, mmHg | 44.9±14.9 | 44.4±14.7 | 53.8±16.8 | 0.004 |
| Calcium score (Agatston) | 2,167 (1,480-2,930) |
2,149 (1,455-2,929) |
2,305 (1,562-3,104) |
0.779 |
| Mitral regurgitation | 0.744 | |||
| Moderate | 92 (14) | 86 (14) | 6 (13) | |
| Severe | 15 (2) | 15 (2) | 0 (0) | |
| Tricuspid regurgitation | 0.715 | |||
| Moderate, n (%) | 59 (11) | 53 (10) | 6 (15) | |
| Severe, n (%) | 3 (1) | 3 (1) | 0 (0) | |
| Systolic pulmonary artery pressure, mmHg | 39.1±13.1 | 39.0±13.2 | 41.7±9.1 | 0.536 |
| Procedural characteristics and in-hospital events | ||||
| Type of valve | 0.557 | |||
| Balloon-expandable | 594 (85) | 551 (85) | 43 (88) | |
| Self-expanding | 106 (15) | 100 (15) | 6 (12) | |
| Valve size, mm | 26 (23-26) | 26 (23-26) | 26 (23-26) | 0.376 |
| Valve-in-valve procedure | 32 (12) | 32 (12) | 0 (0) | 0.182 |
| Procedural approach | 0.741 | |||
| Transfemoral | 651 (93) | 606 (93) | 45 (92) | |
| Transarterial (non-transfemoral) | 49 (7) | 45 (7) | 4 (8) | |
| Predilatation | 89 (21) | 84 (21) | 5 (23) | 0.842 |
| Post-dilatation | 50 (12) | 49 (12) | 1 (5) | 0.279 |
| Major vascular complication | 21 (5) | 19 (5) | 2 (9) | 0.355 |
| Major or life-threatening bleeding | 28 (7) | 27 (7) | 1 (5) | 0.694 |
| Stroke/transient ischaemic attack | 6 (1) | 5 (1) | 1 (2) | 0.351 |
| Length of stay, days | 1 (1-1) | 1 (1-1) | 1 (1-2) | 0.201 |
| Data are presented as mean±standard deviation, n (%), or median (Q1-Q3). NOAF: new-onset atrial fibrillation; Q: quartile; STS: Society of Thoracic Surgeons | ||||
INCIDENCE OF NOAF AND BASELINE CHARACTERISTICS
AECG was started 1 (0-1) day after TAVI and lasted for a median time of 14 (12-14) days. In 49 (7%) patients, NOAF was detected during the AECG (time to NOAF: 2123456 days). Patients were in AF for 185 (43-421) minutes, and the median of the longest registered episode was 138 (48.9-505.6) minutes. AF burden was 0.7% (0.3-2.8) (Figure 1). Only one patient (2%) presented symptoms that could be ascribed to the development of AF (palpitations). However, the 30-day ECG showed no AF.
Baseline characteristics did not differ between patients with or without NOAF, except for higher transaortic peak (no NOAF: 71.9±23.5 mmHg vs NOAF: 85.2±23.8 mmHg; p=0.024), and mean (no NOAF: 44.4±14.7 mmHg vs NOAF: 53.8±16.8 mmHg; p=0.004) gradients (Figure 2). There were no differences between groups regarding procedural characteristics or in-hospital adverse events (Table 1). Baseline and discharge ECG characteristics are detailed in Supplementary Table 1, with no differences between patients with or without NOAF.
At 30-day follow-up, 31 patients (4%) received a permanent pacemaker. Among patients who developed NOAF, 2 (4%) required a permanent pacemaker implantation. In one case, the patient presented a tachy-brady syndrome detected during AECG, and in the other, a complete heart block was detected during monitoring.
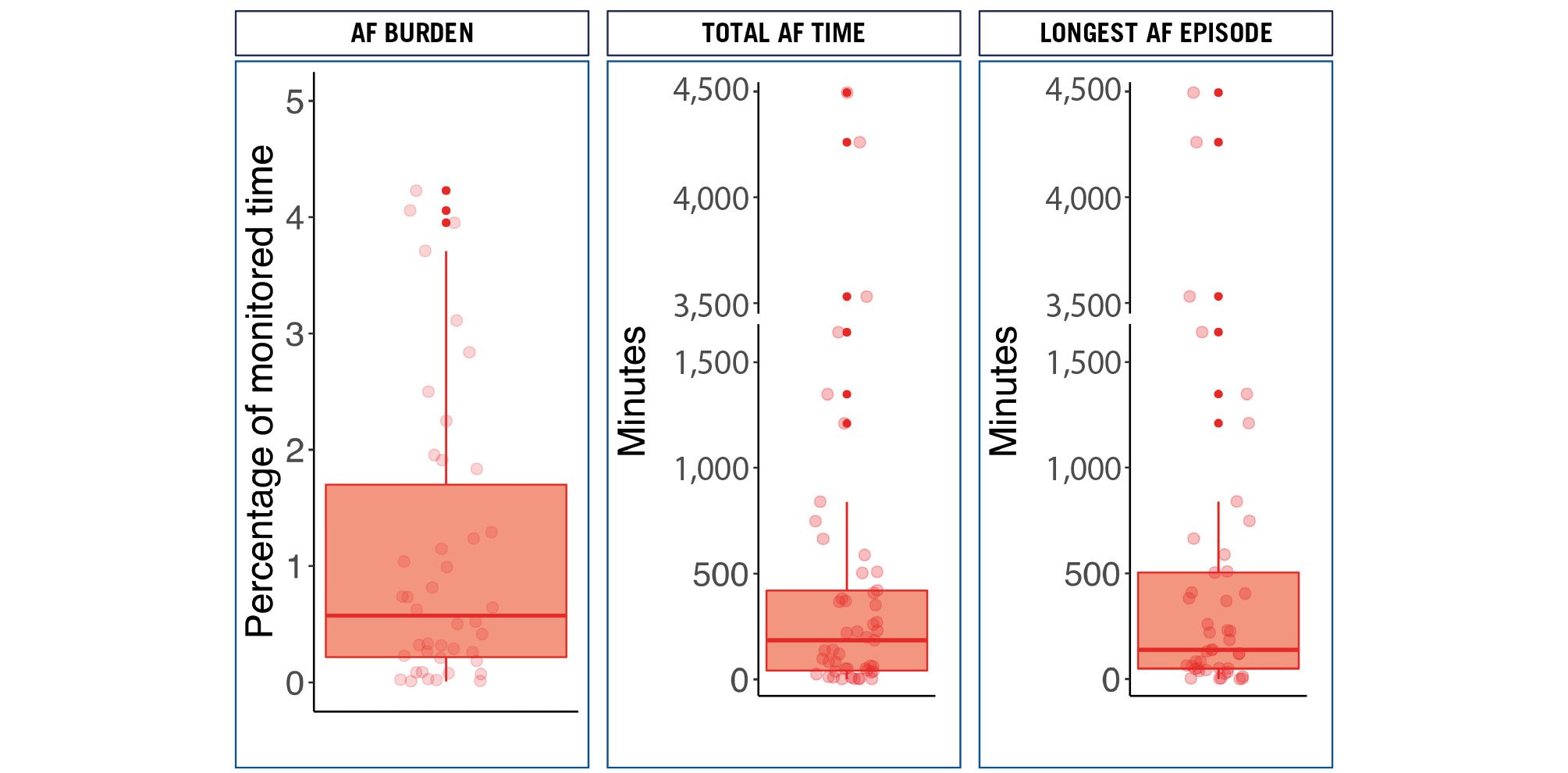
Figure 1. AECG findings: AF burden, total time in AF and duration of the longest AF episode. AECG: ambulatory echocardiogram; AF: atrial fibrillation
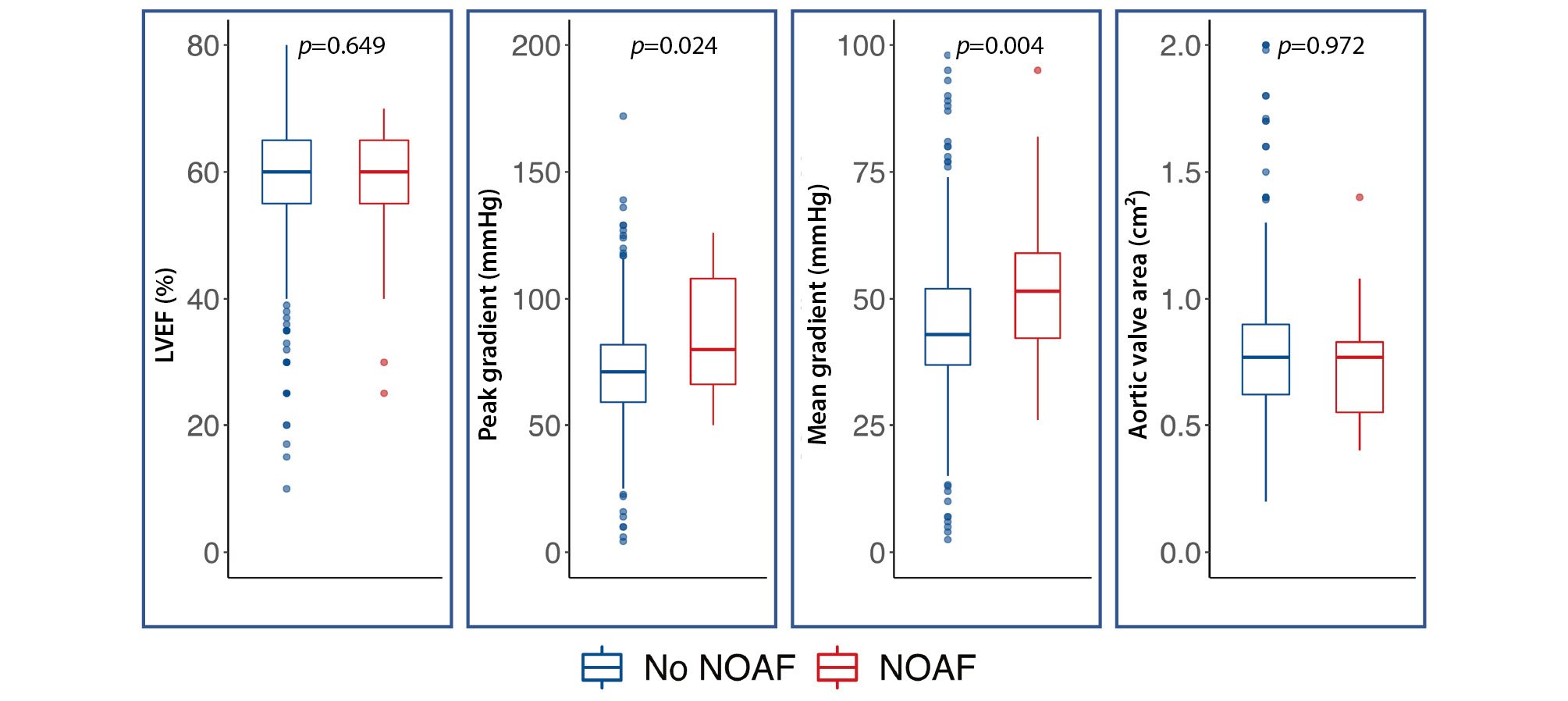
Figure 2. Differences in baseline echo-based parameters between NOAF and no NOAF patients. LVEF: left ventricular ejection fraction; NOAF: new-onset atrial fibrillation
FACTORS ASSOCIATED WITH THE DEVELOPMENT OF NOAF
Table 2 shows the results of the univariable and multivariable logistic regression analyses. In the multivariable model, only the mean aortic gradient was associated with a higher risk of NOAF (odds ratio [OR] 1.04 per 1 mmHg, 95% confidence interval [CI]: 1.01-1.06; p=0.006), while older age showed a trend towards an association with a higher risk of developing subclinical NOAF (OR 1.07 per year, 95% CI: 0.99-1.15; p=0.072).
Table 2. Results of uni- and multivariable logistic regression analysis to identify predictors of NOAF.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, years | 1.04 (1.00-1.08) | 0.072 | 1.07 (0.99-1.15) | 0.072 |
| Female | 1.20 (0.67-2.14) | 0.542 | ||
| Hypertension | 2.33 (0.71-7.67) | 0.163 | ||
| Diabetes mellitus | 0.79 (0.43-1.49) | 0.479 | ||
| COPD | 0.91 (0.40-2.07) | 0.815 | ||
| LVEF, baseline | 1.01 (0.98-1.04) | 0.639 | ||
| Indexed left atrial volume, baseline | 1.02 (0.98-1.07) | 0.364 | ||
| Major vascular complication | 2.02 (0.44-9.30) | 0.364 | ||
| Aortic valve area, baseline | 0.98 (0.31-3.07) | 0.972 | ||
| Aortic gradient – mean, baseline | 1.04 (1.01-1.06) | 0.005 | 1.04 (1.01-1.06) | 0.006 |
| PA systolic pressure, baseline | 1.02 (0.97-1.07) | 0.534 | ||
| Major or life-threatening bleeding | 0.66 (0.09-5.13) | 0.696 | ||
| In-hospital stroke | 2.69 (0.31-23.5) | 0.370 | ||
| Non-transfemoral access | 1.19 (0.41-3.46) | 0.741 | ||
| CI: confidence interval; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; NOAF: new-onset atrial fibrillation; OR: odds ratio; PA: pulmonary artery | ||||
INDICATION OF ANTICOAGULATION IN PATIENTS WITH SUBCLINICAL NOAF
Among the 49 patients in whom NOAF was detected during the continuous AECG, 25 (51%) were prescribed OAC following the documentation of AF. Patients who received OAC had a significantly higher rate of peripheral artery disease (OAC: 8 [32%] vs no OAC: 1 [4%]; p=0.012) but were otherwise similar to patients not receiving OAC in terms of baseline characteristics and stroke risk (assessed by the CHA2DS2-VASc score). Although non-significant, patients started on OAC showed a trend towards a higher AF burden, total AF duration, and longer AF episodes (Table 3, Figure 3).
Table 3. Baseline and AF-related characteristics in patients with NOAF, according to the prescription of oral anticoagulation.
| Total N=49 | No OAC N=24 | OAC N=25 | p-value | |
|---|---|---|---|---|
| Age, years | 80.7±8.5 | 80.8±9.0 | 80.6±8.2 | 0.937 |
| Female | 26 (53) | 12 (50) | 14 (56) | 0.674 |
| Hypertension | 46 (94) | 23 (96) | 23 (92) | 0.576 |
| Diabetes mellitus | 15 (31) | 9 (38) | 6 (24) | 0.305 |
| Coronary artery disease | 27 (55) | 13 (54) | 14 (56) | 0.897 |
| Chronic kidney disease | 13 (27) | 5 (21) | 8 (32) | 0.376 |
| Previous stroke | 5 (10) | 2 (8) | 3 (12) | 0.637 |
| Peripheral artery disease | 9 (18) | 1 (4) | 8 (32) | 0.012 |
| CHA2DS2-VASc score | 4 (3-5) | 4 (4-4) | 5 (3-5) | 0.459 |
| AF burden, % | 0.7 (0.3-2.8) | 0.7 (0.1-1.6) | 1.0 (0.3-3.7) | 0.187 |
| Total time in AF, minutes | 184.7(42.6-420.8) | 138.1(18.8-265.0) | 352.0(51.8-588.7) | 0.139 |
| Longest AF episode, minutes | 138.1(48.9-505.6) | 138.1(25.1-259.9) | 249.5(51.8-664.6) | 0.241 |
| Data are presented as mean±standard deviation, n (%), or median (Q1-Q3). AF: atrial fibrillation; NOAF: new-onset atrial fibrillation; OAC: oral anticoagulation; Q: quartile | ||||
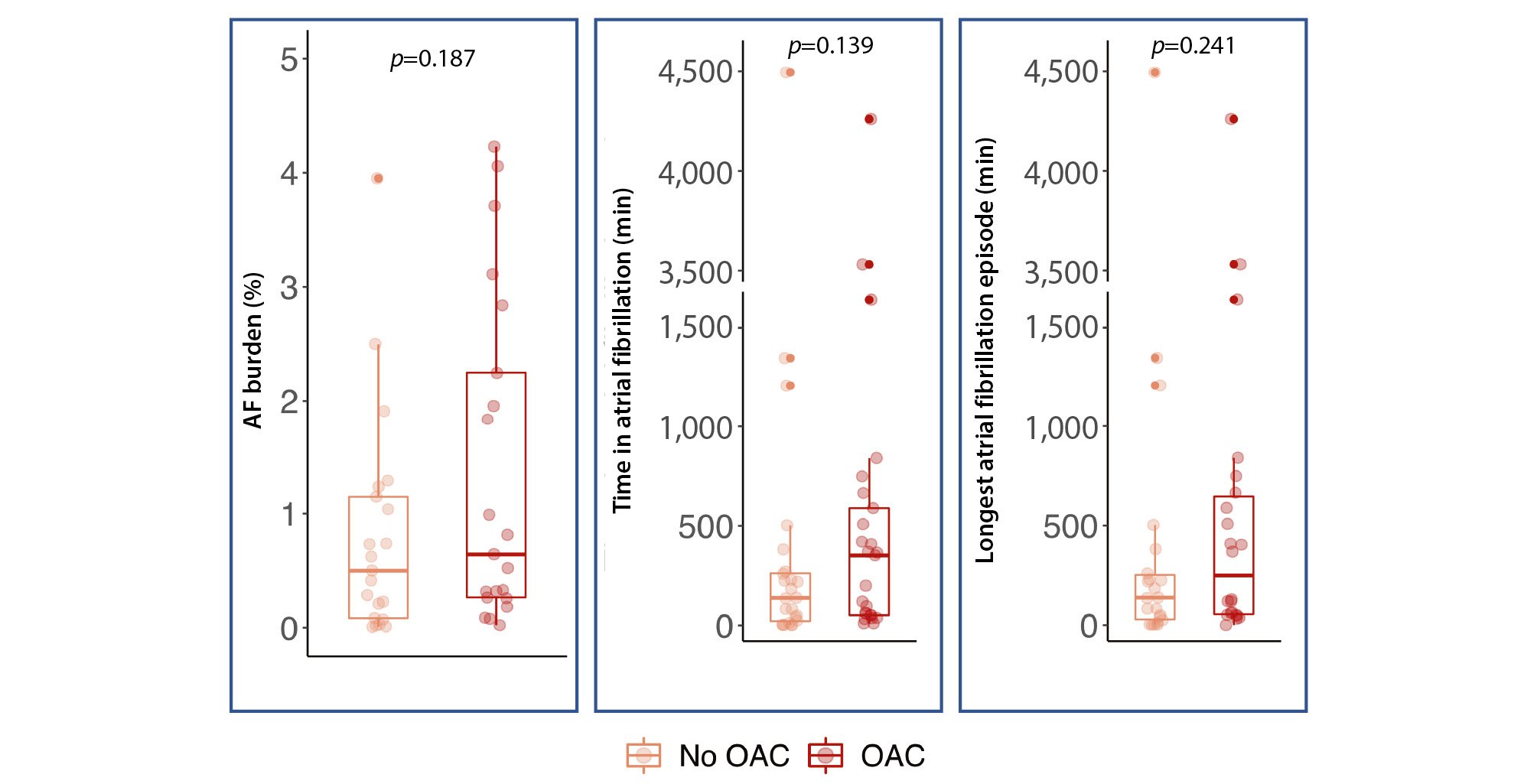
Figure 3. Comparison of AF-related findings between NOAF patients receiving OAC or not. AF: atrial fibrillation; NOAF: new-onset atrial fibrillation; OAC: oral anticoagulation
PROGNOSTIC IMPLICATIONS OF NOAF
The occurrence of all-cause mortality, stroke, death, and combined events for patients with and without NOAF is summarised in Figure 4. Seven patients (1%) died before the 30-day follow-up and were not included in the landmark 30-day to 1-year survival analysis. NOAF was not identified in any of these 7 patients.
The 30-day to 1-year mortality rate for the entire population was 4.3%. There were no differences between groups for mortality (no NOAF: 30 [4.7%] vs NOAF: 0 [0%]; p=0.122), stroke (no NOAF: 9 [1.4%] vs NOAF: 1 [2.0%]; p=0.723; hazard ratio [HR] 1.86, 95% CI: 0.23-15.2), or bleeding events (no NOAF: 12 [1.9%] vs NOAF: 2 [4.1%]; p=0.288; HR 2.23, 95% CI: 0.50-9.98). Nor was there a difference in the occurrence of the combined endpoint of all-cause death, stroke and bleeding (HR 0.91, 95% CI: 0.23-2.05; p=0.963) (Figure 4).
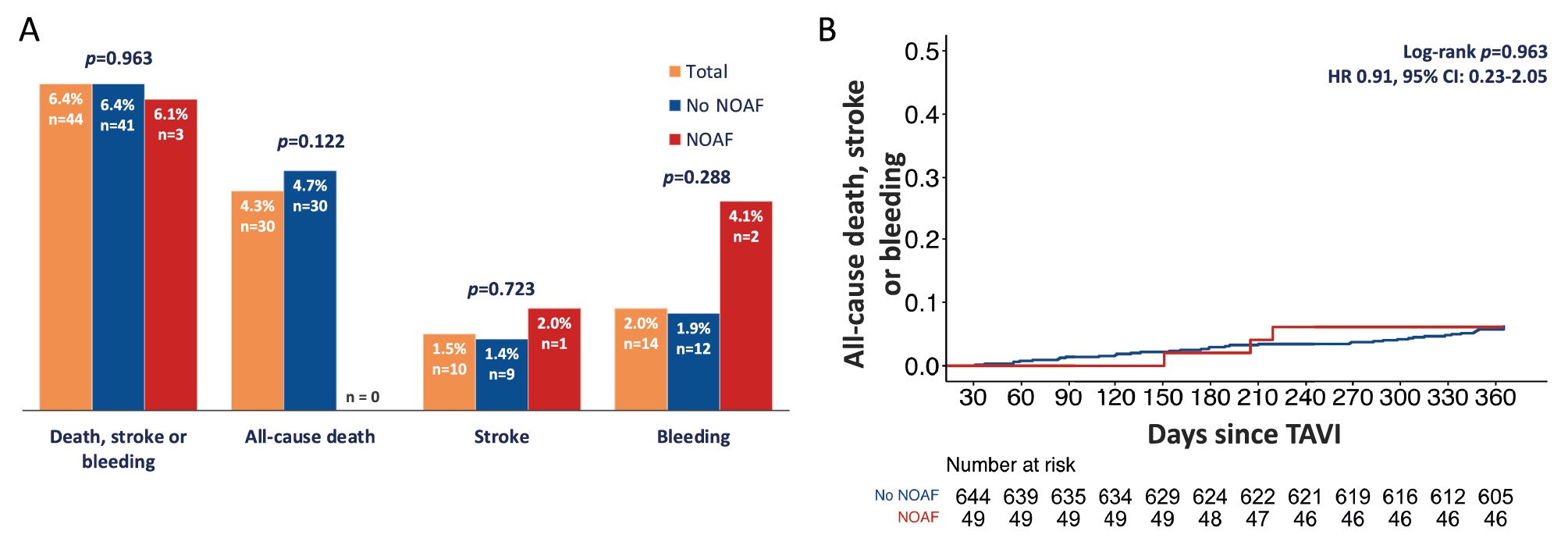
Figure 4. Clinical impact of NOAF in the landmark analysis from 30-day to 1-year follow-up. A) Rate of death, stroke and bleeding events, and the combination of the three in NOAF and no NOAF patients. B) Kaplan-Meier analysis of the combined outcome: all-cause death, mortality, stroke or bleeding. CI: confidence interval; HR: hazard ratio; NOAF: new-onset atrial fibrillation; TAVI: transcatheter aortic valve implantation
Discussion
To the best of our knowledge, this is the first study to address the incidence and clinical implications of subclinical NOAF detected during AECG within the first days after hospital discharge in patients undergoing TAVI. The main results of the study can be summarised as follows (Central illustration): (i) NOAF was identified in close to 1 out of 10 patients, with a median AF duration of about 3 hours and a total AF burden close to 1%; (ii) patients who developed NOAF after TAVI exhibited a higher degree of AS severity; (iii) the antiÂthrombotic management of NOAF episodes was heterogeneous, with only half of the patients receiving OAC after the AECG AF documentation; and (iv) the occurrence of NOAF episodes after TAVI had no impact on clinical outcomes (stroke, mortality, bleeding) at 1-year follow-up.
Central illustration. Incidence, predictors and clinical implications of silent atrial fibrillation after transcatheter aortic valve implantation. AF: atrial fibrillation; CI: confidence interval; ECG: echocardiogram; HR: hazard ratio; NOAF: new-onset atrial fibrillation; TAVI: transcatheter aortic valve implantation
INCIDENCE OF NOAF IN TAVI RECIPIENTS
The typical diagnosis of AF requires ECG tracing ≥30 seconds with no discernible P waves and irregular RR intervals13. The interest in subclinical AF episodes detected by implantable or wearable devices has significantly grown in the last few years. Subclinical NOAF has been detected in 10% of patients receiving a pacemaker within the 3 months following the implant14, and the incidence rises to one-third of patients when the follow-up is extended up to 3 years14.
In most TAVI studies, the diagnosis of NOAF is limited to the index hospitalisation and the 30-day follow-up and is mainly based on in-hospital telemetry monitoring and 12-lead ECG recording at the 30-day follow-up visit3.
Although lower than after surgical aortic valve replacement (SAVR), the incidence of NOAF after TAVI still remains between 7 to 10%3, with a trend towards a lower incidence from the first high-risk population trials to the most recent studies, including low-risk patients15.
Also, observational studies of patients undergoing AECG or receiving a pacemaker after TAVI showed that subclinical NOAF was detected in up to 17% of patients510.
In the general population, the prevalence of AF for patients aged 80 or older is 9%16. In our study, after excluding patients with a history of AF and those who developed in-hospital AF episodes after TAVI, the 14-day incidence of NOAF detected during AECG was 7%. Thus, the TAVI population appears to be a highly vulnerable group with an exceptionally high risk of developing AF.
ATRIAL FIBRILLATION AND AORTIC STENOSIS SEVERITY
Several patient- and procedure-related factors have been shown to be associated with the development of NOAF after TAVI, including older age, worse baseline New York Heart Association Functional Class, lower left ventricular ejection fraction, previous history of cerebrovascular events, dilated left atrium, need for pre- or post-dilatation, periprocedural complications and the transapical approach15. The transÂapical approach shares some common characteristics with SAVR, and the development of NOAF may be associated with procedure-related factors and complications leading to a hyperadrenergic status that may act as an arrhythmic trigger17. In our study, only patients with a transarterial approach were included, with no differences in NOAF development between patients with transfemoral or an alternative transarterial access.
In our cohort, baseline and procedure-related characteristics were well balanced between patients with and without NOAF, except for a higher AS severity in NOAF patients, who exhibited much higher peak and mean transaortic gradients. In addition, the mean transaortic gradient was associated with a higher risk of NOAF in the multivariable analysis. Afterload pressure resulting from AS can lead to electrical remodelling and a high left atrial pressure which could trigger the development of AF18. In fact, in TAVI patients who underwent AECG before the procedure, NOAF or atrial tachycardia was detected in 6%, leading to changes in medical treatment in two-thirds of them19. When symptoms related to AS manifest, they are frequently accompanied by additional cardiac remodelling, including impaired diastolic function, multivalvular disease, pulmonary hypertension, and often AF that could have an impact on survival20.
The possible adverse outcomes associated with a delayed intervention have been recently studied. The AVATAR Trial evaluated the safety and effectiveness of early SAVR in asymptomatic severe AS patients without exertion-induced symptoms, compared to a conservative strategy and surgery based on clinical guidelines. An early surgical intervention reduced the composite outcome of all-cause death, acute myocardial infarction, stroke, or unplanned heart failure hospitalisation. A total of 25 patients crossed over from the conservative treatment group to the surgical group, with a median time from randomisation to surgery of 400 (191-619) days. After the surgery, these patients presented an incidence of NOAF of 20%, while in patients randomised to early surgery, the 30-day incidence of NOAF was 12.5%21. In the field of TAVI, the EARLY TAVR trial (ClinicalTrials.gov: NCT03042104) is currently recruiting asymptomatic severe AS patients to be randomised to TAVI or initial conservative management. It will evaluate the incidence of NOAF as a secondary endpoint. Also, the randomised trials, EXPAND TAVR II (ClinicalTrials.gov: NCT05149755), TAVR UNLOAD (ClinicalTrials.gov: NCT02661451), and PROGRESS (ClinicalTrials.gov: NCT04889872), will evaluate whether patients with symptomatic moderate AS may benefit from early TAVI.
CLINICAL IMPACT AND MANAGEMENT OF SUBCLINICAL EPISODES OF ATRIAL FIBRILLATION
In other studies, NOAF after TAVI has consistently shown to be associated with a higher risk of cerebrovascular events, bleeding, and mortality15. Interestingly, NOAF but not prevÂious AF was associated with a higher incidence of stroke and bleeding in the SOURCE XT Registry and in a large meta-analysis including more than 10,000 patients822. This might reflect the heterogeneity in OAC therapy prescription for postoperative NOAF, constituting an important knowledge gap in postoperative care. The European Society of Cardiology guidelines recommend (Class IIb, Level of Evidence B) long-term OAC for those patients with NOAF after cardiac surgery at risk for stroke, without specifying the duration of NOAF or the CHA2DS2-VASc score that would advise the initiation of OAC13. In TAVI patients, some observational studies showed a beneficial effect of OAC in patients developing in-hospital NOAF, even for patients with AF episodes lasting less than 12 hours323. However, less than 30% of TAVI patients who develop postoperative NOAF are discharged on OAC23.
At the time this study was conducted, evidence on the clinical impact of subclinical episodes of AF detected by implantable or wearable devices was scarce and mainly based on observational studies. In a meta-analysis of 11 studies evaluating the incidence and the impact of implantable device-detected AF, subclinical AF was identified in 35% of patients, and the risk of stroke in patients with subclinical AF was twice that of patients without detected atrial arrhythmic episodes24. The LOOP study randomised patients over 70 years old with an additional risk factor for embolic events and no previous history of AF to undergo or not undergo AECG with an implantable loop recorder to detect AF. The diagnosis of NOAF was higher (31.8% vs 12.2%) in patients undergoing AECG than in the control group (undergoing usual care with an annual interview with the study nurse). This increased the capability of a subclinical AF diagnosis and, consequently, increased OAC prescription (recommended with AF lasting more than 6 minutes), but it did not significantly reduce stroke or systemic embolic events9. In the NOAH trial, treatment with edoxaban (vs aspirin) in patients 65 years or older, with device-detected atrial high-rate episodes lasting ≥6 minutes and who had at least one additional risk factor for stroke, did not reduce the incidence of cardiovascular death, stroke or systemic embolism, as compared with placebo, but did result in a higher incidence of a composite of death or major bleeding25. The ARTESiA trial evaluated the efficacy and safety of apixaban (compared with placebo) in patients with device-detected subclinical AF lasting ≥6 minutes and a CHA2DS2-VASc score of at least 3 points. Patients randomised to apixaban had a lower incidence of stroke and a higher incidence of major bleeding26. A study-level meta-analysis of these trials provided high-quality evidence that OAC reduces the risk of stroke in patients with device-detected AF along with an increase in major bleeding events27.
Before the publication of the aforementioned trials, the cutoff point established for the prediction of stroke varied significantly (from 6 minutes to >5.5 hours) between different studies, which rendered the consensus for a threshold for recommending the initiation of OAC difficult. Nevertheless, an expert consensus document from different international heart rhythm societies recommended OAC for subclinical AF episodes lasting >5.5 hours when the CHA2DS2-VASc score is ≥228. In our study population, the decision to prescribe OAC was left to the discretion of each local Heart Team. Detailed information about the reasons for prescribing OAC, or not, was not available. Patients receiving OAC more often had peripheral artery disease. Also, the AF burden, total time in AF and the duration of the longest AF episode were numerically higher among those patients receiving OAC, although these differences were not statistically significant.
In our study population, despite the high risk for embolic events (median CHA2DS2-VASc score=4), only 51% of NOAF patients received OAC after an AF diagnosis. NOAF was not associated with a higher risk of mortality, stroke, or bleeding. None of the patients with NOAF died during follow-up, and only one had a stroke. In that specific patient, with a CHA2DS2-VASc score of 4, OAC had not been prescribed because of the short duration of the AF episode (4 minutes). Patients with a CHA2DS2-VASc score ≥3 have an elevated risk for stroke, even without AF29. Also, the incidence of late cerebrovascular events in the TAVI population is particularly high30. Thus, the relatively limited size of our study population and the relatively short duration of the follow-up may explain the lack of significant differences in the stroke rate.
USEFULNESS OF AECG FOR THE DETECTION OF NOAF AFTER TAVI
Our study showed that in a population of 700 patients with no previous or in-hospital AF who underwent AECG after TAVI, NOAF was detected in 7% of patients, with a median of 2 days for the occurrence of the first AF episode. Only 50% of patients received OAC after the AF diagnosis, and no differences were found in all-cause mortality or the occurrence of stroke or bleeding. Our experience, based on real-world clinical practice, highlights the heterogeneous management of patients with subclinical AF. Recent evidence showed the potential benefit of OAC in patients with device-detected AF lasting ≥6 minutes. Nevertheless, further efforts are needed to identify patients in whom AECG could be cost-effective for the detection of AF and those who could benefit from OAC, especially in the era of next-day and same-day discharge. Also, the role of wearable devices, which will provide continuous monitoring over longer periods, needs to be addressed15.
Limitations
This study has several limitations that must be acknowledged. The study population comprised patients included in different clinical studies, but also patients who underwent AECG for non-protocolised clinical indications. This, added to the exclusion of all patients with pacemakers prior to TAVI, may have led to a patient selection bias. Also, this is a retrospective analysis of prospectively collected data. The present analysis was not prespecified when local databases were created and completed. Thus, specific information, especially that related to the decision-making process for initiating anticoagulant therapy, was not always available. Patient follow-up was altered because of the COVID-19 pandemic, which might have delayed an intervention in patients in whom NOAF was detected. Patient follow-up was restricted to 1 year, so a higher event rate at longer-term follow-up cannot be excluded. Last, the occurrence of clinical AF during follow-up was not consistently collected in all participating centres, so the incidence of NOAF during the first year in those patients with no NOAF registered during the AECG could not be reported.
Conclusions
Up to 7% of TAVI recipients without a previous history of AF presented NOAF episodes as determined by AECG (2 weeks as the median monitoring time), and those with a higher degree of AS severity had an increased risk. In half of NOAF patients, OAC was initiated, and NOAF was not associated with a higher risk of stroke or mortality. Future studies should determine whether there is benefit of earlier AS interventions, including a potential reduction of NOAF episodes, and evaluate the clinical implications of detecting and treating subclinical NOAF in the high-risk group of TAVI candidates.
Impact on daily practice
Up to 7% of patients undergoing transcatheter aortic valve implantation (TAVI) develop subclinical new-onset atrial fibrillation (NOAF) in the first days after the procedure. Routine ambulatory electrocardiogram monitoring after TAVI may help unmask not only bradyarrhythmic events but also tachyarrhythmic episodes, especially in the era of next-day or same-day discharge. This could also lead to the prescription of oral anticoagulation (OAC) in TAVI patients with subclinical NOAF. Future studies with larger sample sizes and more extended follow-up periods are warranted to determine the clinical impact of subclinical NOAF after TAVI and evaluate the potential clinical benefit obtained from OAC.
Funding
Jorge Nuche is the recipient of a grant from the Fundación Alfonso Martín Escudero (Madrid, Spain). Josep Rodés-Cabau holds the Research Chair "Fondation Famille Jacques Larivière" for the Development of Structural Heart Interventions (Laval University).
Conflict of interest statement
J. Rodés-Cabau has received institutional research grants and speaker/consultant fees from Edwards Lifesciences and Medtronic. I. Nault has received speaker fees from Icentia. A.K. Okoh has received consultant fees from Edwards Lifesciences. M. Russo has received consultant fees from Abbott and Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

