Abstract
Transcatheter aortic valve implantation (TAVI) is an acceptable treatment for patients with aortic stenosis but remains contraindicated for patients with native aortic valve regurgitation (NAVR). It is well established that patients with severe NAVR and symptoms have a poor prognosis if left untreated and that they should be offered surgical aortic valve replacement. There are patients with NAVR and at high surgical risk for whom conventional surgical aortic valve replacement may be unsuitable. Until recently there has been limited experience in the treatment of these patients with TAVI but outcomes from isolated case reports and small registries are encouraging. Of interest are certain new TAVI devices with design features which may make them better suited to the treatment of NAVR patients.
Introduction
Transcatheter aortic valve implantation (TAVI) has developed as the standard of care for inoperable patients with symptomatic severe aortic stenosis, and as an alternative to open surgery in those deemed high risk. Current evidence from randomised controlled trials confirms that TAVI is comparable to surgical aortic valve replacement (AVR) in high-risk patients and has mortality benefits when compared to optimal medical therapy1,2, and appears to be a cost-effective intervention3. Since the first TAVI case was performed in 20024, there have been a steadily increasing number of transcatheter valve implantation procedures performed worldwide, but severe aortic regurgitation remains a contraindication to TAVI.
Native aortic valve regurgitation (NAVR) is an “off-label” indication for transcatheter aortic valve implantation with the current generation of TAVI devices, and the standard of care remains surgical aortic valve replacement (AVR). Despite this, some patients have been treated with TAVI for NAVR, and some next-generation TAVI devices have design features which may make TAVI for this indication a more attractive treatment option in high-risk surgical patients.
Aortic regurgitation
Patients with acute severe NAVR, most frequently caused by aortic dissection or infective endocarditis, have a poor prognosis without intervention due to their haemodynamic instability. Patients with chronic severe NAVR and symptoms also have a poor long-term prognosis5. Severe aortic regurgitation produces a sustained overload on the left ventricle (LV), which compensates by a progressive increase in dimension and hypertrophy, and the left ventricular ejection fraction often remains normal until late in the disease process. Once symptoms become apparent, mortality in patients without surgical treatment may be as high as 10-20% per year and survival rates for untreated patients with NAVR are shown in Figure 16. In asymptomatic patients with severe chronic NAVR and normal LV function, the likelihood of adverse events is low. However, when LV end-systolic diameter (LVESD) is ≥50 mm, the probability of death, symptoms or LV dysfunction is reported to be 19% per year6-8.
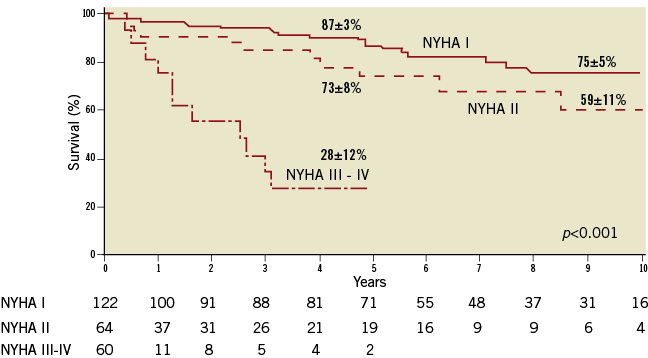
Figure 1. Survival of untreated patients with native aortic valve regurgitation after the onset of symptoms by New York Heart Association (NYHA) class. This figure shows the poor prognosis associated with NYHA Class III or IV symptoms. Figure reproduced with permission from Dujardin et al8.
Causes of NAVR
Unlike aortic stenosis which occurs predominantly as a result of degenerative changes, aortic regurgitation is due to diverse aetiologies. The principal causes of NAVR from the European Heart Survey on valvular heart disease were degenerative, rheumatic, endocarditis, inflammatory, and congenital (Figure 2)10.
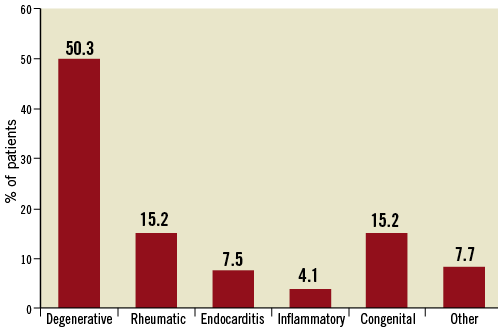
Figure 2. The causes of native aortic valve regurgitation from the Euro Heart Survey of Valvular Heart Disease. Adapted from Iung et al10. Note that the cause of NAVR was degenerative changes in only 50.3% of cases of isolated left-sided heart disease, as compared to aortic stenosis where 81.9% of valve pathology was due to degenerative changes.
Prevalence of NAVR
Asymptomatic NAVR is common. In 1,696 men and 1,893 women (aged 54±10 years) attending a routine examination as part of the Framingham Study, NAVR of more than or equal to trace severity was noted in 13.0% of men and 8.5% of women by echocardiography. In this unselected population, the prevalence of NAVR increased 2.3 times with each decade of life9. In the prospective Euro Heart Survey on valvular heart disease in Europe, aortic regurgitation was present in 369 patients (13.3%) with single native left-sided valve disease. In the same survey, aortic stenosis patients represented 43.1% of patients with single valvular disease10.
Treatments for NAVR
Surgical aortic valve replacement remains the gold standard, but the proportion of valve repair procedures is increasing in experienced centres. Cusp retraction and calcification appear to be the main adverse factors for repair procedures. Operative mortality is low (1-4%) in isolated aortic valve surgery, both for replacement and repair10-16.
The ESC guidelines and ACC/AHA Practice Guidelines recommend AVR for severe aortic regurgitation patients presenting with cardiac symptoms, or left ventricular dysfunction with an EF of <50%4,5. Surgery for asymptomatic patients with severe NAVR and normal LV function should be considered if LV end-diastolic diameter (LVEDD) is >70 mm or LVESD is >50 mm, given that the likelihood of developing irreversible myocardial dysfunction is high if intervention is delayed further, with excellent surgical results if surgery is performed without delay5. Good imaging quality is essential and data confirmation with repeated measurements are recommended before surgery in asymptomatic patients.
Some controversy still exists as to the optimal timing of aortic valve intervention in asymptomatic patients. It should be noted that the evidence used for these recommendations comes from observational studies rather than controlled trials, and this may be a factor in the suggestion that a large proportion of patients with severe NAVR do not receive treatment, as shown in Figure 316.
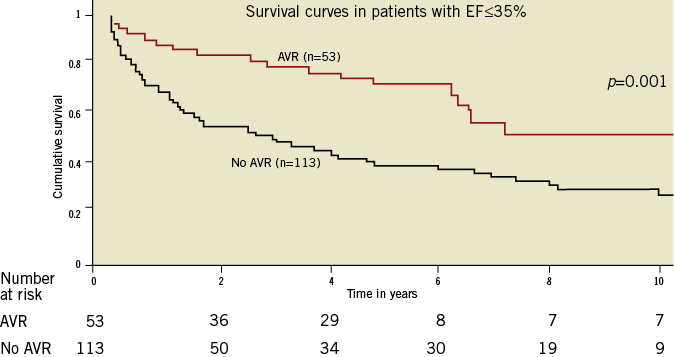
Figure 3. The survival of patients with severe native aortic valve regurgitation and impaired left ventricular function with and without aortic valve replacement. The survival differences question whether many patients with NAVR and impaired left ventricular dysfunction are being undertreated in contemporary surgical practice. Figure reproduced with permission from Kamath et al16.
Transcatheter aortic valve replacement for NAVR
There is limited published clinical experience of treating patients with TAVI for NAVR, and to date there have been no randomised clinical trials. There are a number of reasons why TAVI has not so far been used in larger numbers of patients with NAVR. Firstly, the prevalence of severe aortic regurgitation is lower than that of aortic stenosis, and as a result the initial TAVI devices were designed to treat aortic stenosis. Secondly, although aortic stenosis is predominantly caused by calcification and degeneration of the valve, NAVR is caused by diverse aetiologies and affects younger patients, the majority of whom are clearly surgical candidates. Furthermore, a proportion of patients with NAVR have pathologies involving the ascending aorta, mandating surgical treatment. Finally, patients with NAVR have a more complex and variable anatomy, making transcatheter treatment with the currently available devices more challenging.
A number of isolated case reports describing the use of TAVI for this indication have been described, with successful TAVI implantation in very high-risk patients in whom surgery was not possible18-31. The majority of these reports describe the use of a self-expanding CoreValve® TAVI prosthesis (Medtronic, Minneapolis, MN, USA) in special circumstances. The use of TAVI has also been described for NAVR in patients with a left ventricular assist device (LVAD), where even moderate aortic regurgitation can result in malfunction of the LVAD device23,24,30.
The largest series to date exploring the use of TAVI for NAVR examined 43 patients treated with the CoreValve device for pure NAVR without aortic stenosis32. This was a multicentre registry with 14 contributing centres from Europe and Israel. The patients were extreme risk and had varied aetiologies for NAVR, which are outlined in Table 1. The majority of patients (60.5%) in this study had non-calcified NAVR. Transfemoral access was the preferred access route but subclavian, direct aortic and carotid accesses were also used where there was hostile iliac or femoral anatomy.
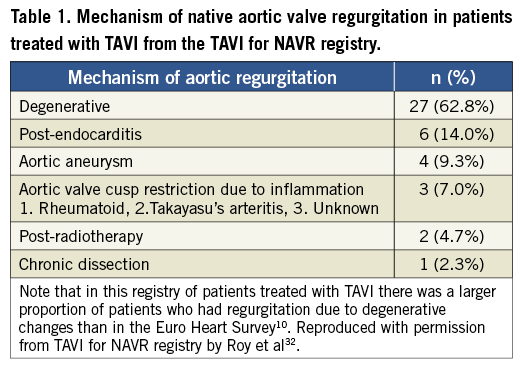
The clinical outcomes of this study according to the Valve Academic Research Consortium (VARC) definitions33 are summarised in Table 2. The 30-day all-cause mortality was 9.3% with a stroke rate of 4.7%, which was encouraging in this extreme risk cohort of inoperable patients, especially when compared with similar inoperable patients from cohort B of the PARTNER trial2. Notably, while implantation of a prosthesis was successful in 97.7% of cases, the VARC-defined procedural success was only 74.4% due to the need for a second valve and ≥ grade II residual aortic regurgitation post procedure. Also of interest was the finding that the need for a second valve was limited to patients without aortic valve calcification. This study demonstrated the feasibility of this technique and highlighted the potential procedural difficulties in treating NAVR with TAVI.
Similar findings were noted in an Italian multicentre registry which examined 26 patients treated with the CoreValve device. A second valve was required in six patients, and three patients were left with > grade 2 aortic regurgitation post procedure34. This registry also compared 30-day mortality in patients treated with TAVI for AS and NAVR, and found a statistically significant difference between the two groups (5.9% for AS and 23% for NAVR). However, given that many AS patients are high-risk but operable, comparing them to inoperable NAVR patients having “off-label” TAVI is difficult.
There have been recent reports with newer-generation TAVI devices for use in NAVR. Seiffert et al described the use of transapical implantation of a JenaValve TAVI device (JenaValve Technology GmbH, Munich, Germany) in patients with non-calcified NAVR. The study included five patients treated with TAVI due to severe comorbidities making surgery unsuitable. All five patients had non-calcified, symptomatic NAVR and one of the patients had moderate aortic regurgitation with severe left ventricular dysfunction and planned LVAD insertion. VARC-2 defined procedure success occurred in all patients with no 30-day mortality or stroke. One patient had acute kidney injury (AKIN) Stage 2, and two patients had AKIN Stage 1, while no patients required a second valve. All five patients also had ≤grade 1 aortic regurgitation post procedure35.
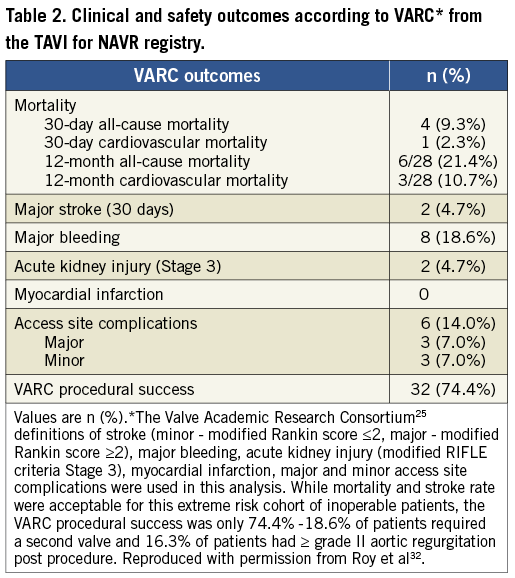
Traditionally, balloon-expandable TAVI devices have not been utilised in “off-label” NAVR, possibly due to lack of calcification at the valve and annulus causing unpredictable deployment, and to concerns regarding annulus rupture in oversizing. However, the concept of using a “dock” system has developed, allowing a more precise and potentially advantageous method of performing TAVI in NAVR. The Edwards HELIO Transcatheter Aortic Dock (Edwards Lifesciences, Irvine, CA, USA) is a novel approach to the treatment of NAVR which uses a pre-placed dock behind the aortic leaflets to facilitate implantation of a SAPIEN XT valve (Edwards Lifesciences). The use of this technique: implanting a transfemoral dock and a balloon-expandable TAVI via the transapical approach has been described by Pasupati et al (Figure 4). Early reports of this technique are promising, and in four patients there was no 30-day mortality with one major stroke and one minor vascular complication36. The first case of using this technique via a transfemoral- transfemoral approach has also been described31. A summary of the experience to date of treating NAVR patients with TAVI is outlined in Table 3.
Figure 4. The Edwards HELIO Transcatheter Dock during transfemoral-transapical TAVI for native aortic valve regurgitation. The native valve leaflets are pinned between the dock and the valve prosthesis, ensuring accurate valve positioning at the annulus and limiting paravalvular regurgitation.
Technical aspects of treating NAVR with TAVI
WORK-UP
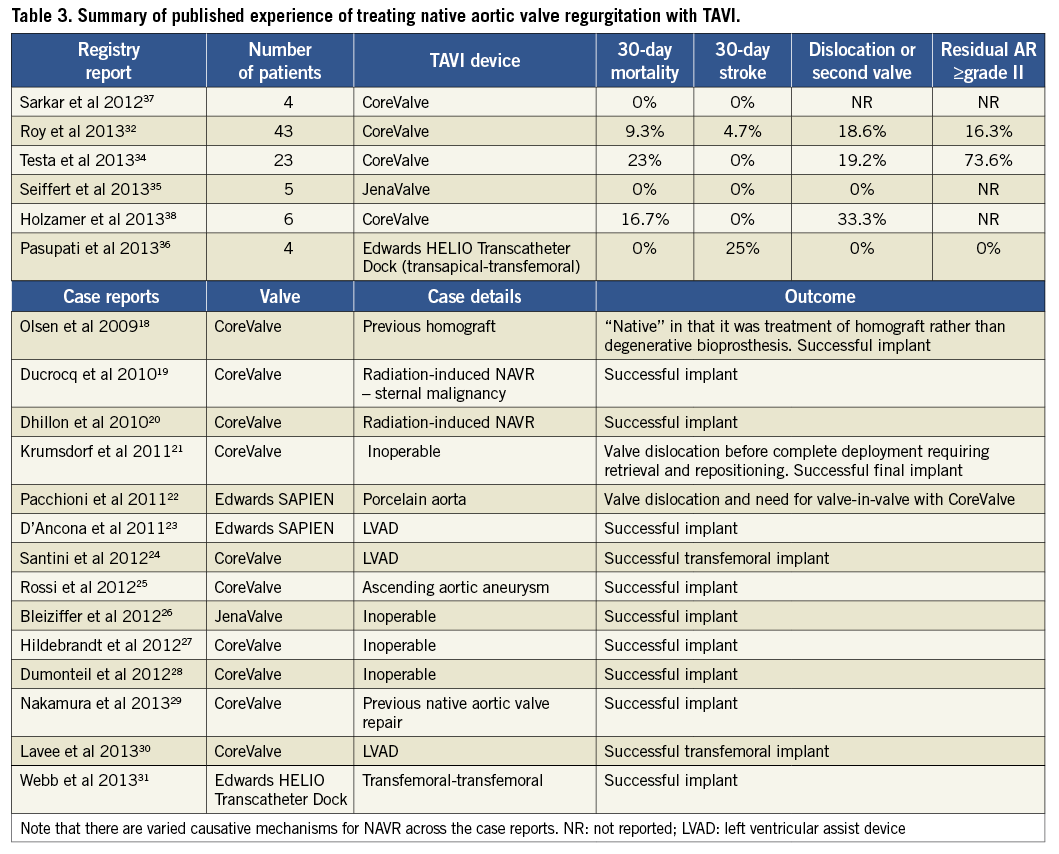
The divers e aetiologies of NAVR can make the anatomy more challenging for TAVI procedures. Patients often have dilatation of the aortic root and ascending aorta, which can make valve deployment with any TAVI device unpredictable. There is a growing body of evidence to support multimodality imaging in all TAVI cases39-41 and, in patients with NAVR, pre-procedure transthoracic echocardiography, transoesophageal echocardiography and multidetector computed tomography (MDCT) should be considered essential. MDCT in particular provides advantages not only because it outlines the entire aortic anatomy but also because of its utility in valve sizing. Figure 5 shows an example of a work-up MDCT prior to TAVI for NAVR.
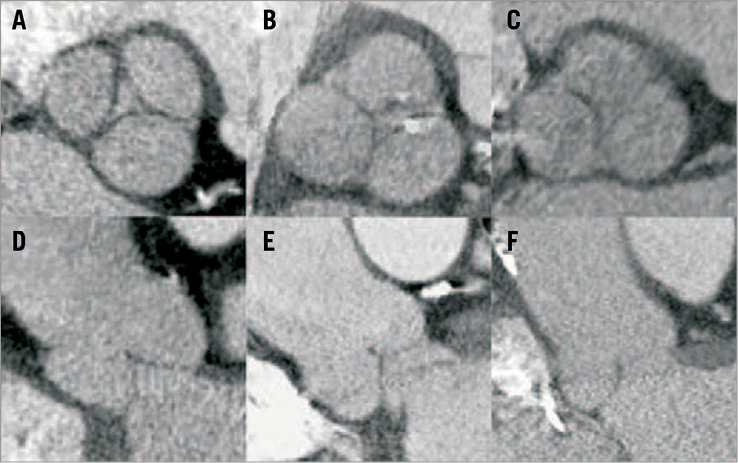
Figure 5. Multidetector computed tomography (MDCT) in the work-up of 3 patients with native aortic valve regurgitation. Multiplanar reconstruction in axial views at the level of the sinuses of Valsalva (A,B,C) and corresponding coronal views (D,E,F). Apart from accurate annulus sizing, MDCT provides essential additional information regarding the sinuses and ascending aorta which is not always clear from echocardiography. Multimodality imaging should be considered an essential step in the preparation and planning of TAVI procedures for patients with NAVR. Image reproduced with permission from Seiffert et al35.
Valve sizing should be performed by careful multiplanar MDCT examination of the annulus in systole, according to annulus perimeter and area rather than minimum and maximum orthogonal diameters39. When valve sizing is being performed for TAVI with a self-expanding prosthesis, at least 10-20% oversizing with respect to the annulus is recommended32.
ACCESS
While transfemoral access is often preferred due to a perception of its being less invasive, direct aortic and transapical access may offer advantages in valve stability during difficult deployments. Specific device requirements and the patient’s own anatomy should be taken into account when deciding which access route to use for the TAVI procedure in NAVR patients32.
TAVI FOR NAVR PROCEDURE
Lack of fluoroscopic landmarks can make valve deployment more difficult in patients with absent aortic valve and annulus calcification. While there are useful computer software programmes integrating CT and fluoroscopy to aid valve deployment, these programmes use algorithms which rely on fixed aortic calcification landmarks and may not be of specific use in NAVR cases. The use of operator-defined fixed landmarks in the thoracic anatomy, such as vertebral bodies, pacing wires, and sternal wires may be utilised and correlated with set-up aortography. Another technique is to use two pigtail catheters, with one placed in the non-coronary sinus and the other placed in the left sinus to define the aortic annulus as demonstrated in Figure 6. These techniques are useful to ensure accurate valve positioning and to avoid valve dislocation or excessive use of contrast.
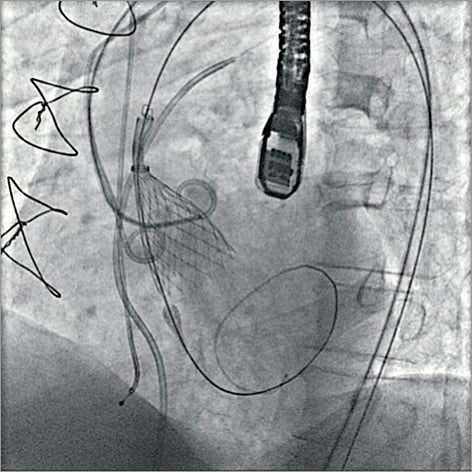
Figure 6. Two-pigtail technique for outlining the aortic annulus. In patients with NAVR who have no valve calcification, the procedure can be more challenging due to lack of fluoroscopic landmarks to outline the annulus position and root anatomy. The two-pigtail technique can be used to define the annulus with a pigtail placed in the non-coronary and left coronary sinuses during the TAVI procedure. Reproduced with permission from Roy et al32.
With respect to the self-expanding CoreValve device, rapid pacing, which is often not required in AS cases, can be useful. Rapid pacing reduces the regurgitant volume as well as the systolic blood pressure and reduces the risk of prosthesis movement and dislocation. The use of rapid pacing from the 1/3 - 2/3 deployment phase is shown in Figure 7.
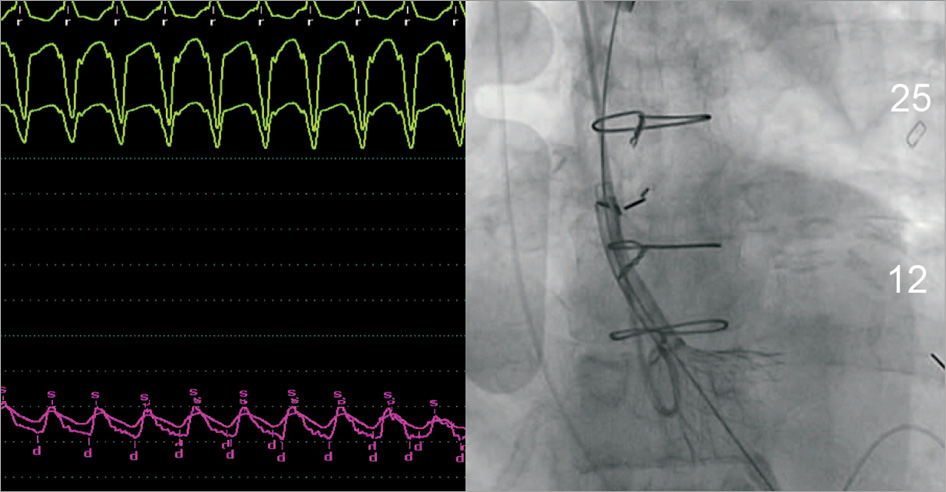
Figure 7. Rapid ventricular pacing during valve deployment. This shows rapid pacing used in the 1/3 - 2/3 deployment phase during TAVI for NAVR with the CoreValve prosthesis. Rapid pacing reduces regurgitant volume and reduces movement of the prosthesis during deployment. Unlike balloon-expandable valves, in TAVI procedures using self-expanding valves for aortic stenosis, rapid pacing is not routinely used during valve deployment. Reproduced with permission from Roy et al32.
Future TAVI devices for NAVR
While there is clinical experience in using TAVI for NAVR, the currently available TAVI devices were specifically designed for the treatment of aortic stenosis. New-generation valve designs that use leaflet “pinning” or aortic “docking” may demonstrate greater efficacy for this indication, but clinical trials and registries are necessary.
The Medtronic Engager™ (Medtronic) (Table 4) was initially developed as a transapical system but is also being developed as a direct aortic system. The Engager is a flexible prosthesis composed of three bovine pericardium leaflets sewn to a polyester sleeve and mounted on a compressible, self-expanding nitinol frame. A main feature of the valve is commissural support arms intended to release and engage against the sinuses, allowing for correct anatomical positioning and axial fixation, with the goal of minimising paravalvular leakage. It has supra-annular valve function which ensures uncompromised valve function even in elliptical annuli. It should be noted that this device has been developed for the treatment of aortic stenosis but the “pinning” of the valve leaflets may also be a design advantage when treating patients with NAVR as it prevents valve migration and minimises paravalvular regurgitation.
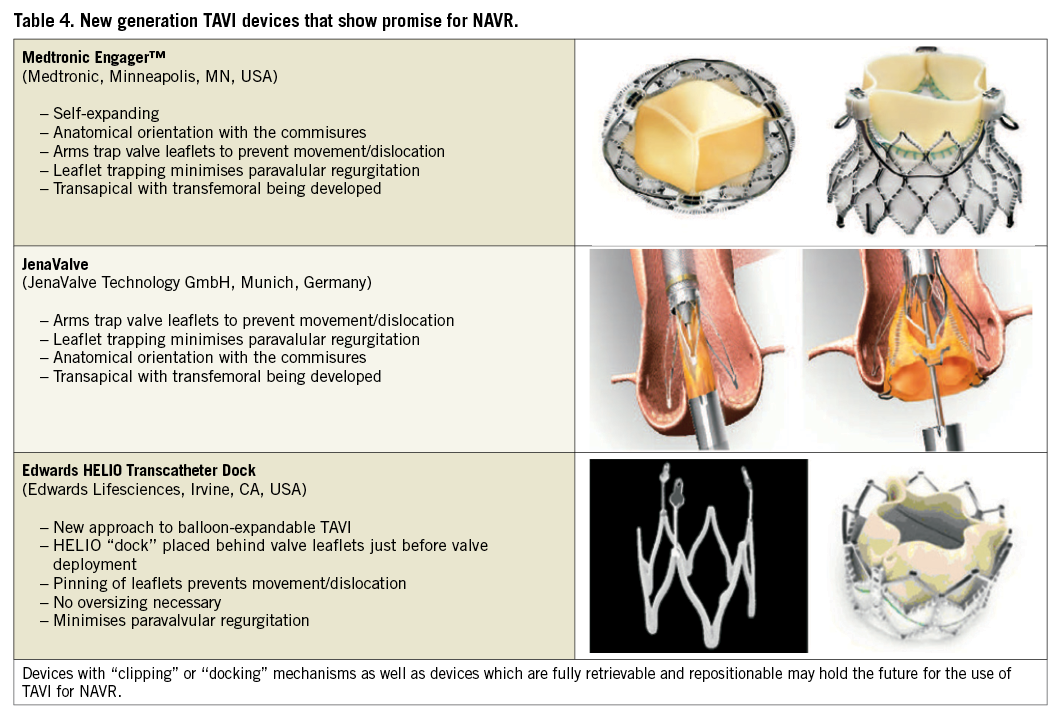
The JenaValve is another transapical TAVI device with an anchoring and clipping mechanism which allows the patient’s native valve leaflets to be clipped to the valve enabling the valve to be firmly anchored in the correct anatomical position and to prevent migration. The JenaValve prosthesis consists of a natural aortic porcine root bioprosthesis fitted with an outer porcine pericardial patch, a so-called skirt, before being sewn onto a nitinol self-expanding stent. Like the Engager it is retrievable and repositionable, which are attractive features for use in NAVR. The JenaValve is displayed in Table 4 and early experience with its use for NAVR has been described above35.
The Edwards HELIO Transcatheter Aortic Dock is a new concept that allows the use of a balloon-expandable valve (SAPIEN XT) to be inserted within a pre-inserted aortic dock ring. The advantages in the treatment of NAVR are similar to those of self-expanding devices that use valve “pinning”, as the dock effectively allows for trapping of the native valve leaflets between the dock and the valve. This ensures valve stabilisation and prevents migration while minimising paravalvular regurgitation. Early experience with this dock and valve via the transapical-transfemoral and transfemoral-transfemoral approaches have been encouraging and are described above. Table 4 shows the design of the dock device and Figure 4 shows the device in use during a transfemoral-transapical case.
Limitations of TAVI for NAVR
While high-risk surgical and inoperable patients with NAVR may be suitable candidates for TAVI in the future, there are certain patients for whom TAVI is not likely to be an acceptable treatment option. Acute regurgitation, most commonly caused by infective endocarditis or dissection, is unlikely to be an indication for TAVI and most often necessitates urgent surgery. Aneurysmal dilatation of the ascending aorta with annulus dilatation causing NAVR is also unlikely to be a suitable pathology for treatment with TAVI, as definitive treatment for these patients includes surgery on the ascending aorta.
Summary
TAVI is now an acceptable treatment option for high-risk surgical patients with severe symptomatic aortic stenosis and the treatment of choice for inoperable patients. Many patients with mixed aortic valve disease with severe stenosis and at least moderate regurgitation have been successfully treated with both balloon-expandable and self-expanding TAVI, but NAVR without stenosis is still considered a contraindication in published guidelines. Despite this, TAVI has been used in patients with NAVR in small numbers with acceptable results. While diverse aetiologies of NAVR may lead to uncertainty concerning the use of TAVI for this indication, the lack of calcification may lead to less paravalvular regurgitation. It is also worth noting that, because of the different aetiologies of valvular disease in patients with NAVR as compared to aortic stenosis, the outcomes should not be directly compared, either in clinical outcomes from trials or in VARC-defined outcomes. It may be necessary in the future to adjust VARC-240 outcomes to include specific measures that relate to NAVR patients.
New devices with valve “pinning” or “docking” techniques are worth exploring and may open this indication for the treatment of high surgical risk patients. What is essential is that, before TAVI for NAVR can be adopted more widely, larger registries and ultimately randomised controlled trials need to be completed.
Conflict of interest statement
S.J.D. Brecker receives consultancy fees from Medtronic. D. Roy and R. Sharma have no conflicts of interest to declare.

