Abstract
Aims: The main cause of acute myocardial infarction (AMI) is the disruption of a thin-cap fibroatheroma (TCFA) and subsequent thrombosis. Mortality increases in diabetic patients due to cardiovascular events; there may be differences in the vulnerable plaques between diabetic and non-diabetic patients. We used optical coherence tomography (OCT) to assess the incidence of vulnerable plaques in diabetic patients with AMI.
Methods and results: OCT was performed in all three major coronary arteries of 70 AMI patients: 48 non-diabetic and 22 diabetic patients. The OCT criterion for TCFA was the presence of both a lipid-rich plaque composition and a fibrotic cap thickness of <65 µm. A ruptured plaque contains a cavity in contact with a lumen and a residual fibrous cap. OCT identified 68 plaque ruptures (1.0 per patient; range, 0-3) and 162 TCFAs (2.3 per patient; range, 0-5). The incidences of plaque rupture and TCFA at culprit lesions were similar. However, non-culprit-lesion TCFAs were observed more frequently in diabetic patients than in non-diabetic patients.
Conclusions: Although the prevalence of vulnerable plaque in culprit lesions was similar between diabetic and non-diabetic patients, vulnerable plaques were observed in non-culprit lesions more in diabetic patients than in non-diabetic patients.
Introduction
Atherosclerotic cardiovascular disease, in particular coronary heart disease, is a major cause of morbidity and mortality in patients with diabetes mellitus (DM)1. DM is generally considered to be a potent risk factor for the development and progression of coronary atherosclerosis. Furthermore, late mortality rate is higher in diabetic patients than in non-diabetic patients among those who have survived acute myocardial infarction (AMI)2,3. Immediately following an AMI, the prognosis that they will suffer in-hospital mortality due to congestive heart failure is nearly twice as high for diabetic patients4,5. The high late mortality rate seen in diabetic patients following an AMI is primarily related to recurrent myocardial infarction6,7. Recently, it has been shown that there are multiple vulnerable plaques besides the “culprit” stenosis in individual patients with AMI8,9, and that the presence of multiple vulnerable plaques is associated with an increased recurrence of acute coronary events leading to AMI8. Other groups showed that plaque morphology is closely related to the plaque disruption and thrombosis, leading to AMI10,11. Specifically, the precursor for later ruptures is likely to be characterised by a large lipid core with an overlying thin fibrous cap measuring <65 µm in thickness, i.e., thin-cap fibroatheromas (TCFAs)12.
Unfortunately, TCFAs are not accurately assessable with current imaging modalities such as angiography, intravascular ultrasound and angioscopy, due to their insufficient resolution or lack of penetration into the plaque. Optical coherence tomography (OCT) is an optical analogue of intravascular ultrasound that allows high resolution (approximately 10 µm) tomographic imaging, and is used to assess TCFAs reliably and reproducibly13,14. The current study employed OCT to assess whether there is a difference in the incidence of complex plaque morphology, including TCFA, between diabetic and non-diabetic AMI patients.
Methods
STUDY POPULATION
A prospective but non-consecutive series of 92 patients with suitable vascular anatomy, who were scheduled for primary percutaneous coronary intervention (PCI) to treat AMI, underwent OCT examination of all three major epicardial coronary arteries. In this OCT study, ST-segment elevation AMI patients (ST-segment elevation of at least 0.1 mV in two or more continuous electrocardiographic leads) and non-ST-segment elevation AMI patients were included. AMI was defined as continuous chest pain at rest with abnormal levels of creatine kinase-MB. Patients with a history of myocardial infarction, a history of coronary artery bypass grafts (CABG), and severe left ventricular dysfunction were not enrolled because of the potential difficulty in acquiring and interpreting OCT images with such conditions. Identification of culprit lesions involved the combination of left ventricular wall motion abnormalities, ECG findings, and angiographic lesion morphology13,15. Patients were considered as having DM if they were on medical treatment for DM or fasting blood sugar level ≥126 mg/dl. The population was divided into two groups according to the presence or absence of DM. The institutional review board approved the study, and all patients provided written informed consent before participation.
OCT IMAGING PROTOCOL
All OCT examinations were performed after intracoronary administration of 3-5 mg isosorbide dinitrate. An over-the-wire occlusion balloon catheter (OBC) (Helios; LightLab Imaging Inc., Westford, MA, USA) was advanced over a 0.014-inch angioplasty guidewire to the culprit lesion and the lesion was crossed with the catheter. The guidewire was then removed from the OBC inner lumen and a 0.016-inch imaging wire (ImagingWire; LightLab Imaging Inc., Westford, MA, USA) was inserted through the OBC. With the ImagingWire held in place across the culprit lesion, the OBC was withdrawn until the balloon was positioned proximal to the culprit lesion. Then, the occlusion balloon was inflated to between 0.4 and 0.6 atm while lactated Ringer’s solution was infused from the distal tip of the OBC at 0.5 ml/sec to flush blood from the imaging field. An imaging run was performed from the distal segment through the lesion to the proximal segment of the culprit lesion using automated transducer pullback at 1.0 mm/sec. After the evaluation of the OCT image of the lesion, PCI with stenting was performed in the usual manner such that TIMI grade 3 flow was achieved.
Following PCI treatment of the culprit lesion, OCT examinations of all three major coronary arteries were performed. The arteries were imaged continuously over a 20 to 50 mm length from the distal segment to the ostium. Normally three to five OCT pullbacks were repeated step by step to image one coronary artery. Only the most proximal 3 mm segments of the arteries were not imaged by OCT as that segment was obscured by the occlusion balloon. During this procedure, ST-segment elevation, patient symptoms, and haemodynamics were observed carefully.
OCT ANALYSIS
OCT images were analysed using proprietary software from LightLab Imaging, Inc. (Westford, MA, USA) by two independent observers who were blinded to the clinical presentations using validated criteria for plaque characterisation13,16. Qualitative OCT plaque composition assessment was performed for each plaque. Distinction of two separate plaques in the same artery (i.e., culprit versus non-culprit lesion) required a ≥5 mm non-atherosclerotic reference segment dividing them; otherwise, it was considered to be one long lesion17.
The size of an OCT-identified lipid lesion was quantified as the number of quadrants displaying the lipid pool on the cross-sectional OCT image. When lipid was present in ≥1 quadrant in any of the images within a plaque, it was considered a lipid-rich plaque. For each plaque categorised to be lipid-rich, the cross-sectional still frame image displaying the most quadrants subtended by the lipid pool was selected for analysis. For all plaques containing a lipid pool, the covering fibrous cap was measured at its thinnest part five times, the minimum and maximum values neglected, and the average of the three remaining values computed to represent the fibrous cap thickness. For OCT image analysis, we defined a TCFA as a lipid-rich plaque with a fibrous cap ≤65 µm at its thinnest point13 (Figure 1A). A plaque rupture was defined as a plaque containing a cavity which was in contact with the lumen which had any overlying residual fibrous cap fragment (Figure 1B).
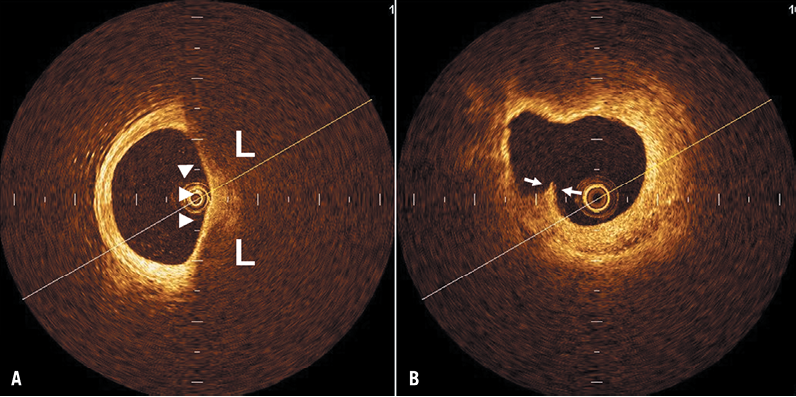
Figure 1. Representative optical coherence tomography images of a thin-cap fibroatheroma (TCFA) and a plaque rupture. Lipid-rich (L) plaque covered by a thin fibrous cap (arrowheads) indicates a TCFA (A). Plaque rupture contains a cavity with an overlying residual fibrous cap fragment (arrows) (B).
ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiography was analysed using a computer-assisted, automated edge-detection algorithm (CMS; Medis, Leiden, The Netherlands) using standard measurements by an independent observer who was blinded to clinical and OCT information. An MLD=0.0 was assumed in the presence of a total occlusion.
Statistics
Continuous variables were reported as mean±SD. The unpaired student’s t-test was used to test the differences between two sets of data with normal distributions. If normality tests failed, the Mann-Whitney U test was used. Categorical variables were reported as frequencies and compared using chi-square statistics. To evaluate interobserver agreement for TCFA, all OCT images were reviewed and a subset of plaques was classified by a second observer and the κ statistic was calculated. Agreement between observers for characterising TCFA was good (κ=0.88) in this study. A p value of <0.05 was considered statistically significant.
Results
BASELINE PATIENT CHARACTERISTICS
Of the 92 enrolled patients, 15 patients did not undergo three-vessel OCT examinations because of left main or severe three-vessel disease. Four were excluded from analysis because TIMI grade 3 flow was not achieved after PCI treatment of the culprit lesion. Three patients were also excluded from OCT image analysis because one or two coronary arteries were not successfully imaged by OCT due to arterial tortuosity or stenosis. Accordingly, a total of 70 patients with AMI were prospectively included in the study.
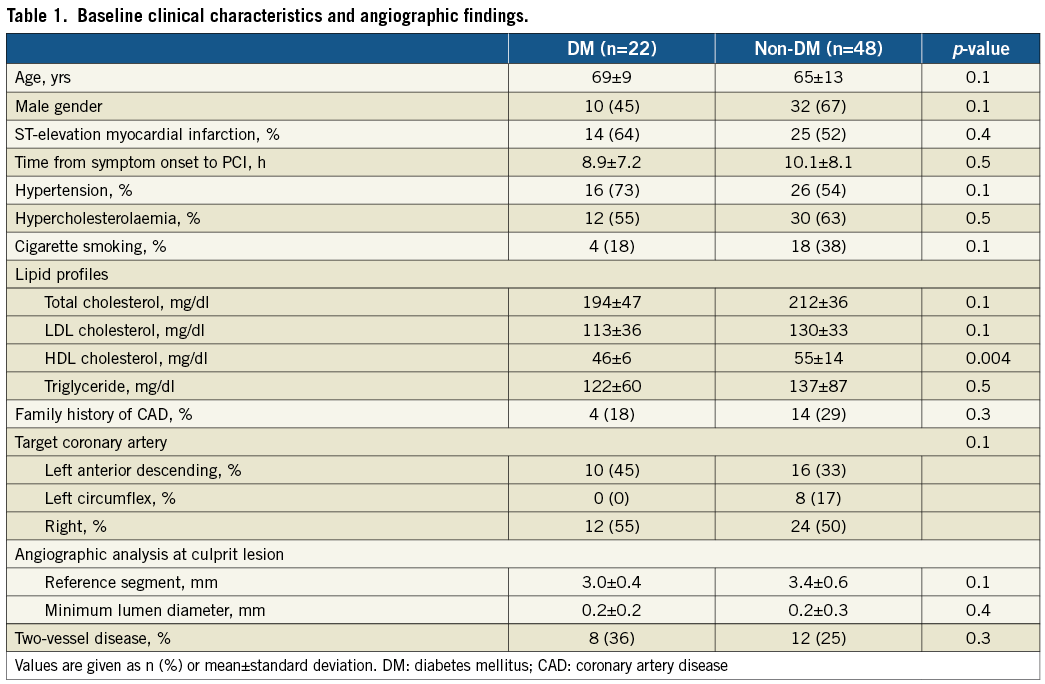
The average time from symptom onset to PCI was 5.9±3.8 hours for ST-elevation AMI and 14.8±13.8 hours for non-ST-elevation AMI (p<0.01). There were 48 non-diabetic and 22 diabetic patients. Of the 22 diabetic patients, six were on diet therapy, 12 were treated with oral hypoglycaemic drugs, and only four required insulin therapy. Baseline clinical characteristics and angiographic findings are shown in Table 1. There was no significant difference between diabetic and non-diabetic patients in baseline clinical characterisation and angiographic findings except for a higher level of serum HDL cholesterol in non-diabetic patients. In addition, there were no significant differences in patient characterisation between insulin-treated diabetic patients and non-insulin-treated diabetic patients. However, diabetic patients requiring insulin treatment had significantly higher baseline percent haemoglobin A1C (13.8±3.2 vs. 6.8±1.1, p<0.001) and tended to have higher baseline plasma fasting glucose level (169±29 vs. 141±9.0 mg/dl) than the diabetic patients treated without insulin.
OCT FINDINGS
The total length of all three coronary arteries imaged by OCT was similar between diabetic and non-diabetic patients: 76±32 vs. 85±29 mm in the LAD, 58±31 vs. 69±28 mm in the LCX, and 96±43 vs. 107±42 mm in the RCA. Sixty-eight plaque ruptures (mean 1.0 per patient; range 0-3) and 162 TCFAs (mean 2.3 per patient; range 0-5) were identified by OCT. The mean value for the TCFA minimum fibrous-cap thickness was 57.9±6.3 µm in diabetic patients and 56.3±5.8 µm in non-diabetic patients (p=0.2). The location of TCFAs was similar between diabetic and non-diabetic patients (proximal: 43% vs. 38%; middle: 33% vs. 45%; and distal: 24% vs. 17%, respectively). The distribution of plaque ruptures was also similar between diabetic and non-diabetic patients (proximal: 50% vs. 44%; middle: 20% vs. 32%; and distal: 30% vs. 24%, respectively). Although the mean numbers of plaque ruptures per patient were similar between the two patient groups (1.0±1.0 for diabetic vs. 1.0±0.9 for non-diabetic, p=0.9), the total number of TCFAs was significantly larger in diabetic patients than in non-diabetic patients (3.1±1.2 vs. 2.0±1.3, p<0.001) (Figure 2). The incidences of plaque rupture and TCFA within the culprit lesion were similar between the two groups. Among the 70 patients, however, 54 (77%) had at least one TCFA discovered in a non-culprit lesion: 100% (22/22) in diabetic patients and 67% (32/48) in non-diabetic patients (p=0.002). Similarly, 22 (31%) had at least one plaque rupture somewhere other than the culprit lesion: 27% (6/22) in diabetic patients and 33% (16/48) in non-diabetic patients (p=0.6). Percentage distribution of TCFAs and plaque ruptures other than those within the culprit lesion is shown in Figure 3. Multiple TCFAs were identified in 20 diabetic patients (91%) and 28 non-diabetic patients (58%) (p=0.006), and multiple plaque ruptures were identified in six diabetic patients (27%) and 14 non-diabetic patients (29%) (p=0.8).
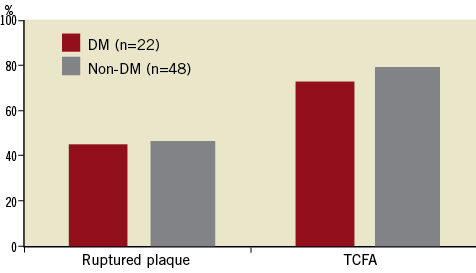
Figure 2. The frequency of plaque rupture and thin-cap fibroatheroma (TCFA) detected in the culprit lesion between diabetic and non-diabetic groups. No significant differences existed between the two groups.
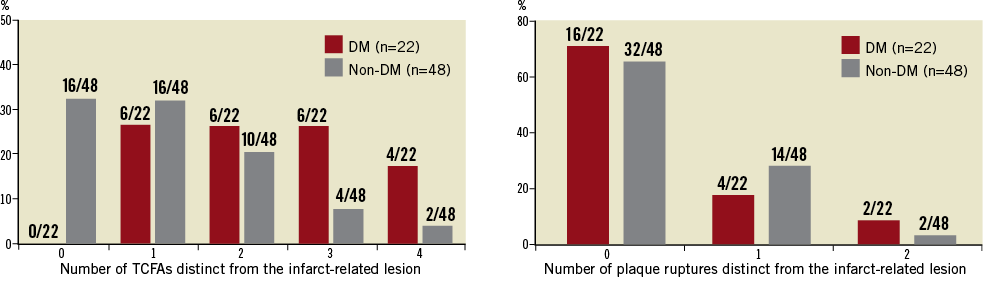
Figure 3. Percentage distribution of coronary thin-cap fibroatheromas (TCFAs) and previous plaque ruptures detected in non-culprit lesions. In 77% of cases, at least one TCFA was found. Similarly, in 31% of patients, at least one plaque rupture was identified.
Discussion
Mortality from myocardial infarction is markedly higher in diabetic patients than in non-diabetic patients18,19. Despite an improvement in the management of patients with acute coronary syndromes, the syndrome is still linked to a substantially increased morbidity and mortality among diabetic patients, as demonstrated by the OASIS and GUSTO II trials20,21. Since the prevalence of DM in patients with AMI is high, and the prevalence in the general population is expected to increase during the coming decades22, management of diabetic patient cohorts will substantially affect total morbidity and mortality rates in coronary heart disease.
Previous studies have reported that plaque rupture occurs not only within AMI culprit lesions but also in non-culprit lesions in patients with various clinical presentations23, suggesting that some plaque ruptures lead to clinical manifestations whereas others remain asymptomatic and heal with subsequent fibrosis. However, DM is characterised by elevated concentrations of procoagulant factors and decreased concentration of antithrombotic factors24. Corti et al25 identified significant reductions in tissue factor activity and blood thrombogenicity in diabetic patients with improved glycaemic control. These observations highlight the concept of high-risk blood in the pathophysiology of diabetic atherosclerosis leading to coronary thrombosis. Therefore, diabetic patients who have coronary atherosclerosis comprising TCFAs, which are known to be precursor lesions vulnerable to plaque rupture, may have a higher risk of coronary thrombosis following plaque rupture compared with non-diabetic patients. Thus, it is clinically important to improve glycaemic control for the reduction of the incidence of adverse cardiovascular events.
It has been reported that insulin resistance is associated with macrophage upregulation of CD36 protein with an increased uptake of oxidised low-density lipoprotein26. Hyperglycaemia increases macrophage matrix metalloproteinase expression27, which is known to play a major role in atherosclerosis and plaque instability by remodelling the extracellular matrix28. In addition, matrix metalloproteinase 2 is a marker for microangiopathy in children with type 1 diabetes29, suggesting a pivotal role for macrophages in the pathogenesis of diabetic atherosclerosis. These findings may affect the plaque instability not only at the culprit lesion but also throughout the entire coronary tree and therefore also non-culprit lesions.
In the present three-vessel OCT study, the overall frequency of TCFA was 2.3 per patients with AMI. In accordance with the current study, Hong et al30 reported that there was an average of 2.5 virtual-histology-derived TCFAs per patient with acute coronary syndrome in a three-vessel virtual histology intravascular ultrasound study. In a pathologic study by Kolodgie et al31 there was an average of three TCFAs in male patients dying with acute myocardial infarction and/or acute plaque rupture. The different frequency of TCFA in the pathologic and OCT studies may be because of different diagnostic methods, clinical presentations or demographics of the study population, number of study patients, and definitions of a TCFA. Furthermore, recently Feng et al32 reported that there was no difference found in the frequencies of vulnerable plaque, including TCFA, plaque rupture, and plaque erosion, between diabetic and non-diabetic patients with unstable angina pectoris. This discrepancy between the previous OCT study and the current study can be explained by differences in methods. In the current study, OCT examinations were performed in all three major coronary arteries even if an angiogram did not show stenosis in the non-infarct-related artery. On the other hand, only one artery was imaged by OCT in most cases in the previous OCT study. Another explanation for the discrepancy may be differences in population sample and selection.
Limitations
This study was a single-centre study with a relatively small study population. Further multicentre studies are required to confirm the results in a larger population of patients. Although the study was performed prospectively, the use of pre-intervention OCT and the decision to perform three-vessel OCT imaging were at the discretion of the operator. Some ruptured plaques with small cavities may have evaded detection by OCT, especially when the cavity was filled by thrombus at the culprit site. The proximal 3 mm of the coronary arteries were not imaged by OCT due to the need to occlude via proximal balloon inflation. In the current study, patients with triple vessel disease were excluded because of the potential difficulty in acquiring and interpreting OCT images. Therefore, some selection bias may be inherent. Finally, as mentioned above, we did not attempt three-vessel OCT examinations when patients were on cardiogenic shock, high-grade AV block, and had severe renal failure. Therefore, approximately one third of STEMI patients were not thought to be eligible for enrolment in the study. OCT examinations were attempted only when the patients’ haemodynamic parameters were stable, and non-culprit arteries were imaged after the successful treatment of culprit lesions. Furthermore, ST-segment elevation, patient symptoms, and haemodynamics were observed carefully during the OCT procedure. It is necessary to establish safety, so they must be performed carefully.
OCT of non-culprit vessels in patients with a recent myocardial infarction may be associated with the potential risk of complications. Therefore, only experienced operators performed these procedures in well-selected patients with a favourable anatomy.
Conclusion
Although the prevalence of vulnerable plaque in the culprit lesion was similar between diabetic and non-diabetic patients, diabetic patients had a larger number of vulnerable plaques which had developed elsewhere compared to non-diabetic patients. This difference suggests an increased vulnerability for plaque rupture leading to coronary thrombosis in patients with diabetes mellitus.
Acknowledgements
The authors thank the staff in the cardiac catheterisation laboratory in Hyogo College of Medicine, Nishinomiya, Japan for their excellent assistance during the study.
Conflict of interest statement
The authors have no conflicts of interest to declare.

