
Abstract
Aims: The purpose of this registry was to determine the long-term outcomes in patients with asymptomatic contained annulus rupture (CR) as a rare complication of transcatheter aortic valve implantation (TAVI).
Methods and results: The ENCORE registry is a multicentre registry (six centres across Europe) of patients with CR diagnosed on post-TAVI computed tomography angiography (CTA) or transoesophageal echocardiography (TEE). A total of 21 patients (mean age 81.9±4.1 years, 81% balloon-expandable TAVI prostheses) were diagnosed with CR (mean size of lesions was 15.3±6.9 × 8.5±3.3 × 8.5±2.3 mm). Seventeen were diagnosed among a total of 1,602 consecutive routine post-TAVI CTA (incidence 1.1%), two in TEE and two in post-TAVI CTA (each conducted due to suspicion of peri-interventional complications). During a mean follow-up of 2.3±1.7 years (cumulative 48.6 patient-years), nine patients (43%) died from non-cardiac causes. None of the patients exhibited symptoms or underwent interventional treatment related to the CR; no sudden cardiac death occurred. A follow-up CTA, performed in eleven patients 240±176 days post TAVI, revealed stable CR findings in seven, regression in one, and remission in three patients.
Conclusions: The results of our international multicentre registry demonstrate favourable long-term outcomes of CR after TAVI supporting a watch-and-wait strategy in these patients.
Introduction
One of the most threatening complications of TAVI is the rupture of the aortic root or annulus, with an estimated incidence of 0.9%1,2. Besides immediately clinically apparent ruptures, asymptomatic contained ruptures of the aortic annulus (CR) have been diagnosed as an incidental finding on computed tomography angiography (CTA) performed after TAVI, with an incidence of up to 5%3. Risk factors for the occurrence of CR are severe transcatheter heart valve (THV) oversizing and increased calcification of the device landing zone2,3.
However, information on outcomes in the event of CR is scarce and limited to the direct post-interventional period or at longer-term follow-up in case reports2,4,5,6,7. Recently, we published an international case collection with long-term observation for a follow-up period of 2.5+1.5 years, in which all patients remained asymptomatic without specific treatment8. These results supported a wait-and-watch strategy in patients with CR. However, our data were limited because of a restricted sample size (twelve patients from three TAVI centres) and the fact that all patients were treated with balloon-expandable THVs.
Therefore, the purpose of this study was to determine the long-term outcomes in patients with CR from a larger international, multicentre registry.
Methods
REGISTRY DESIGN
The ENCORE registry (EuropeaN COntained RupturE registry) was designed to collect data from centres across Europe having cases with CR. The registry was not supported by any external funding. This retrospective analysis included patients with TAVI performed before October 2017. A total of six centres across Europe (three in Germany, two in Denmark and one in the United Kingdom) contributed all their CR cases to the registry. The 12 patients with a CR from our previously published international case collection were re-enrolled in the ENCORE registry with a renewed follow-up8.
A CR, diagnosed on post-TAVI CTA or transoesophageal echocardiography (TEE), was defined as a peri-annular pseudo-aneurysm formation with connection to the intra-aortic lumen, in contrast to an intramural haematoma with the integrity of all vascular wall layers (Figure 1). Pseudo-anonymised data collection was performed via dedicated case report forms including baseline, peri-interventional, and clinical follow-up data at pre-specified time points (one to five years). In cases of death or development of cardiovascular symptoms, the information was evaluated for a possible connection to the CR by two independent cardiologists.

Figure 1. Illustration of the definition of a contained rupture in post-TAVI CTA. Contrast-enhanced retrospectively ECG-gated CTA axial reconstructions of the device landing zone showing a contained rupture as a peri-annular pseudo-aneurysm formation (brace) with the integrity of all vascular wall layers and a connection to the intra-aortic lumen (<-> double arrow).
TAVI PROCEDURE
In all patients the eligibility for TAVI, the preferred access route and the valve size selection were determined by institutional multidisciplinary Heart Teams, based on either preprocedural TEE or CTA imaging. The procedure was performed via a transapical or transfemoral access using standard techniques9. THV implantations were mainly performed under general anaesthesia using fluoroscopy guidance. At the time of the intervention, all patients gave their written informed consent for the anonymised use of clinical, procedural, and follow-up data.
CTA IMAGE ACQUISITION
The pre- and post-TAVI CTAs were performed with different scanner models (mostly SOMATOM Definition Flash or Force; Siemens Healthcare, Forchheim, Germany) and centre-specific scanning protocols. In most of the cases, a retrospective ECG-gated data acquisition was performed by means of bolus tracking. The amount of iodinated contrast agent varied between 50 and 100 mL, depending on the centre and the timing of scanning (pre- or post-TAVI). For the most part, raw data were reconstructed in 50 ms or 5% steps throughout the cardiac cycle.
Four of the participating centres performed routine post-TAVI CTA in all eligible patients with a median interval of 8.5 [IQR 4.25; 12] days after the procedure, usually pre-discharged between day 2 and day 12 after TAVI. This was performed according to the guidelines after thoracic aortic stent implantation10. The intention was to identify possible complications, e.g., aortic injuries, thrombosis of the valves, underexpansion of the prosthesis.
STATISTICAL ANALYSIS
Continuous variables are reported as mean and standard deviation (SD). Categorical variables are reported as frequencies and percentages. All statistical analyses were performed using SPSS software, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 21 patients with a contained rupture post TAVI were identified between July 2008 and October 2017. In the four centres performing routine post-TAVI CTA, CR were delineated in 17 out of the 1,602 (1.1%) patients (Figure 2). In one of these cases, a haemodynamically relevant pericardial effusion had to be drained during the TAVI procedure, but the patient was asymptomatic at the time of the CR diagnosis on post-TAVI CTA. The periprocedural echocardiographic monitoring of the remaining 16 patients was unremarkable.
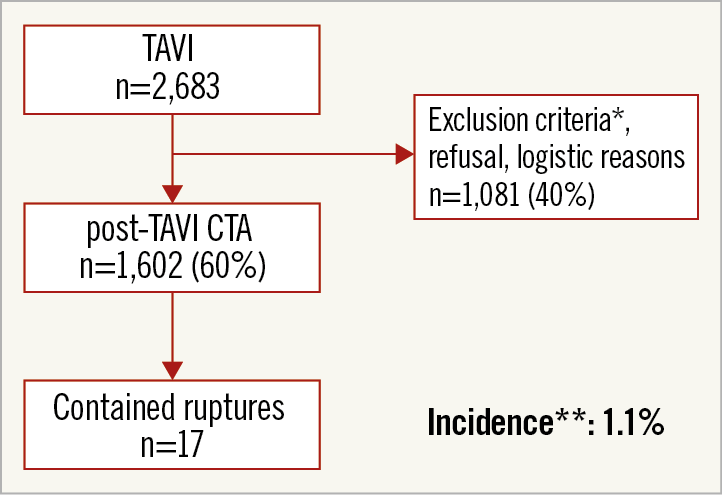
Figure 2. Flow chart of patients who underwent TAVI within the centres of the registry with routine post-TAVI CTA. * Exclusion criteria were: acute renal failure or presence of severely impaired renal function (creatinine clearance <30 ml/min), post-TAVI severely reduced general state of health. ** Incidence of contained ruptures of the aortic annulus within the patients who underwent post-TAVI CTA.
In two of the other four patients, periprocedural TEE or transthoracic echocardiography (TTE) revealed findings suggestive of an aortic intramural haematoma and a pericardial effusion. Due to these findings, post-TAVI CTA was conducted which demonstrated a CR with questionable causal association to the initial findings. The CR of the remaining two patients was delineated in periprocedural and post-procedural TEE.
For the overall cohort, the mean interval (SD) from TAVI procedure to diagnosis was 16±15 days. The baseline echocardiographic and computed tomography characteristics are summarised in Table 1. The mean age of the patient cohort with CR was 81.9±4.1 years; there were 18 females (86%) with a mean logistic EuroSCORE of 12.9±5.1 and STS score of 4.4±1.4%. The majority of the cases occurred after choosing a transfemoral access route (n=17; 81%) or implantation of balloon-expandable valve types (n=17; 81%). A post-dilatation was performed in each of the four CR occurring after the implantation of self-expanding THVs. The mean THV oversizing (based on the annular area) was 23.0±11.4%.
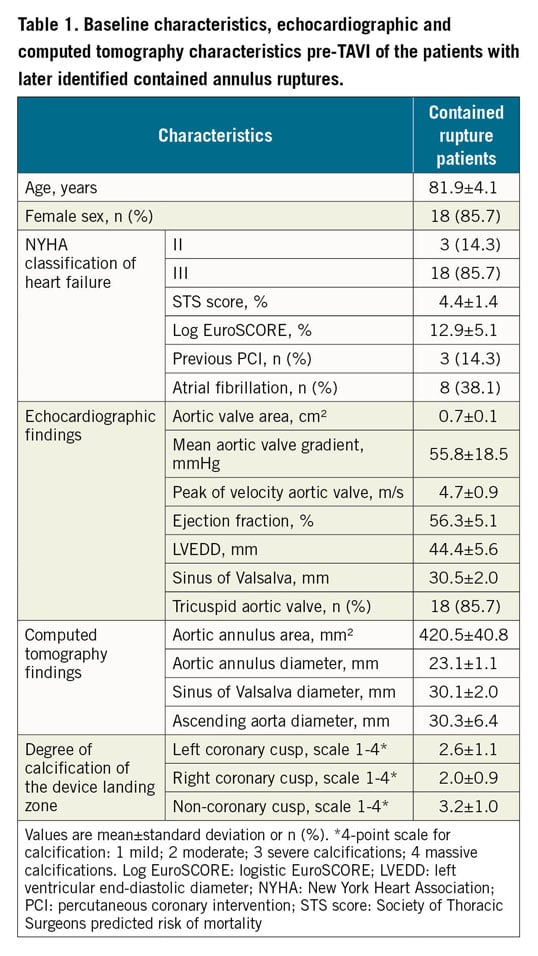
In pre-TAVI CTA, the highest amount of calcification of the device landing zone was found next to the non-coronary cusp (Table 1), whereas the CR were located in 13 (62%) of the cases adjacent to the left coronary cusp (Table 2). The mean size of the lesions was 15±7×9±3×9±2 mm with a maximum size of 24×17×14 mm, resulting in a cranial shift of the left main stem (Figure 3, Table 2). Only the case with the largest CR was detectable by TTE. During the index hospital stay, none of the patients was symptomatic or received any specific treatment for CR. Table 2 summarises the periprocedural characteristics as well as in-hospital events.
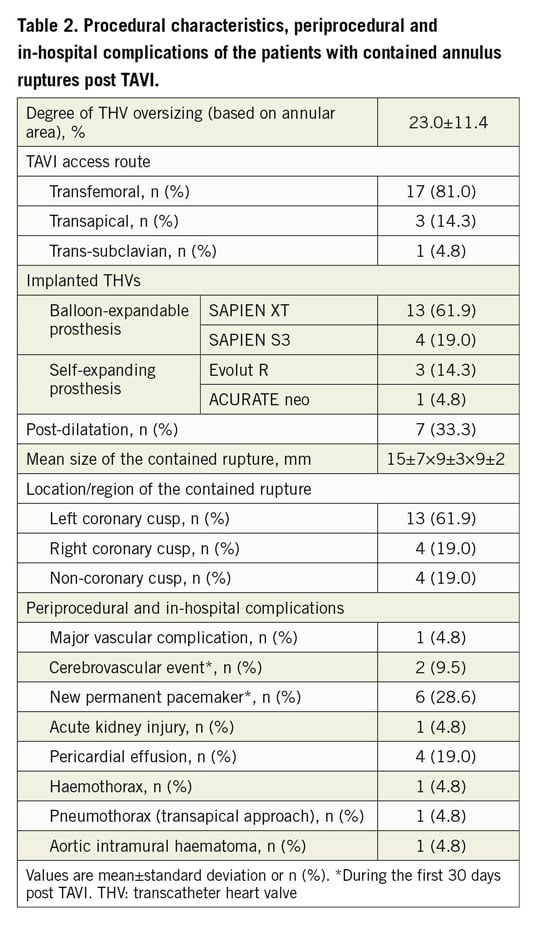
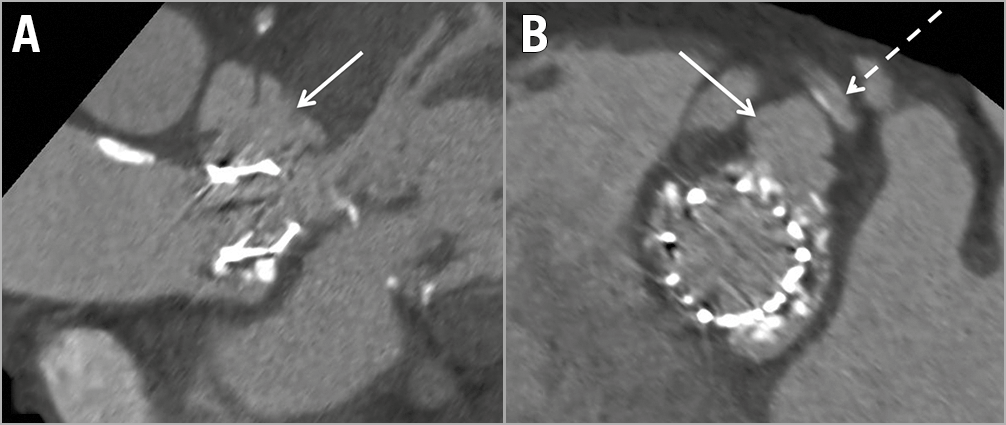
Figure 3. Pronounced finding of a contained rupture leading to a left main stem shift. Contrast-enhanced retrospectively ECG-gated CTA axial and sagittal oblique reconstructions showing a contained rupture of the aortic annulus (→ arrow in A, B) nine days after transcatheter aortic valve replacement with the maximum detected size of 24×17×14 mm in an 84-year-old female. The contained rupture led to a cranial shift of the left main stem (---> dashed arrow in B).
ANTITHROMBOTIC TREATMENT
In 19 of the 21 cases the antithrombotic was not altered after diagnosis of CR. Thus, standard dual antiplatelet therapy (DAPT) was administered in 12 patients, and seven patients with atrial fibrillation received antiplatelet monotherapy plus anticoagulation for the first six to twelve months depending on concomitant percutaneous coronary intervention. One patient received aspirin monotherapy after CR diagnosis instead of DAPT, and one patient with atrial fibrillation received warfarin solely without aspirin.
FOLLOW-UP
The mean follow-up time of the overall cohort was 2.3+1.7 years (cumulative 48.6 patient-years) with a maximum observation length of 5.1 years (1,843 days). During the follow-up period, none of the 21 patients exhibited symptoms, underwent interventional treatment or died related to the CR. None of the patients died through a sudden cardiac death. Nine patients (43%) died from non-cardiac causes (e.g., acute kidney or respiratory failure, infection). At least one follow-up CTA was performed in 11 (52%) patients with a mean time to last follow-up CTA of 240+176 days. These follow-up CTAs revealed stable CR findings in seven (64%), regression in one (9%), and a complete remission in three patients (27%) (Figure 4).
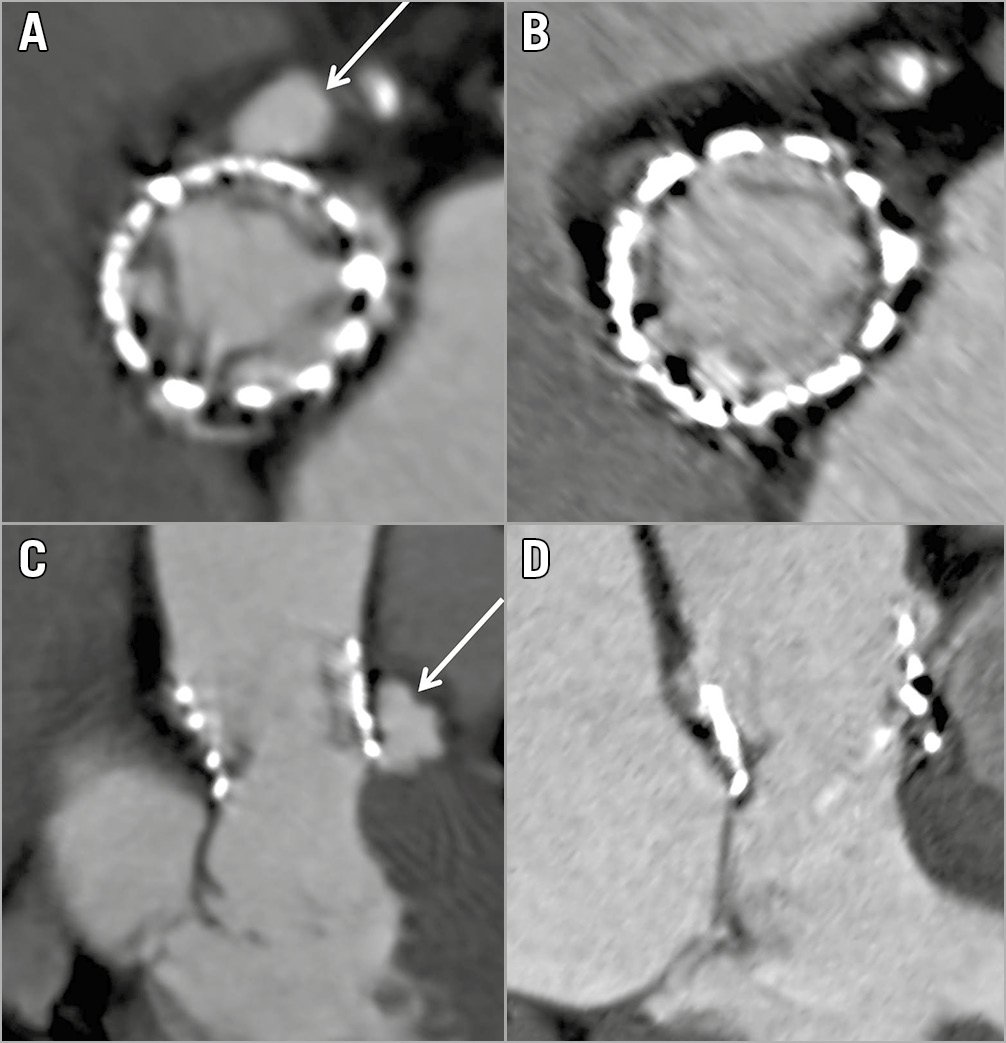
Figure 4. Complete remission of a contained rupture during follow-up. Contrast-enhanced retrospectively ECG-gated CTA axial and sagittal oblique reconstructions showing a contained rupture (arrow in A, C) of the aortic annulus in a 77-year-old female 15 days after transcatheter aortic valve replacement (A, B). The follow-up CTA after eight months showing a complete remission of the contained rupture (C, D). Differences between the CTA images (A and B vs C and D) regarding the adjacent structures are caused by a different time point of image reconstruction throughout the cardiac cycle (for the Figures we chose the time point with the fewest artefacts).
Discussion
Our study is the first to report the long-term outcomes in patients with post-TAVI CR in a large, international multicentre registry. Our results suggest a favourable course of this complication over a mean follow-up period of 2.3+1.7 years.
Short-term follow-up studies, case reports with follow-up of up to six months and a recently published cases series demonstrated favourable outcomes of CR2,3,6,7. The current registry adds to these studies by providing CR longer-term follow-up in a large series of consecutive patients having CTA performed following TAVI with various valve designs. Thus, we were able to expand the information on the benign nature of contained rupture substantially compared with these previous observational studies.
Two previous case reports describe a complicated course of contained ruptures. Erez et al reported on a patient with contained rupture resulting in compression of the left main coronary artery, treated by percutaneous intervention4. Himbert et al published a case of CR with sudden death of unknown cause within the first month post TAVI11.
In contrast to these reports, all our patients were asymptomatic during in-hospital stay and no specific treatment was necessary. All patients remained asymptomatic during the follow-up period. In 11 of our patients with at least one additional follow-up CTA, CR sizes were stable findings, demonstrating partial or complete regression. This is in accordance with our previously published study, where we observed stable CTA findings in 60% of the patients and complete regression in 40% after a comparable follow-up period8.
CR of the aortic annulus after TAVI with balloon-expandable THVs have been described previously with an estimated incidence of approximately 5% and are commonly present as an incidental finding on post-TAVI CTA3. The lower incidence in our study compared with that of Blanke et al (1.1% vs 5.0%) may be explained by differences in crucial determinants of outcome between studies, e.g., cohort size (1,602 vs 65 patients), a changed THV design with newer valve generations and more knowledge about risk factors for CR over time3. The majority of cases (62%) in the ENCORE registry occurred after the implantation of an earlier generation of balloon-expandable THVs (SAPIEN XT; Edwards Lifesciences, Irvine, CA, USA). The sample size and the registry design of our study did not allow reliable analyses of predictors of contained ruptures. Nevertheless, it is tempting to speculate that the risk for CR diminishes by using new-generation THVs, more optimal THV sizing and growing experience about the TAVI procedure.
In accordance with formerly identified and described predictors of aortic root injury, the mean prosthesis oversizing was >20% in our cohort and we identified pronounced calcifications in the left ventricular outflow tract, in particular adjacent to the non-coronary cusp2,3. Furthermore, all patients with a CR after implantation of a self-expanding THV underwent peri-interventional post-dilatation. Even if the registry was not designed to analyse risk factors, one might assume that avoiding post-dilatation may prevent CR, especially in patients with self-expanding prostheses.
Consistent with previous reports, most (61.9%) of these contained ruptures were located adjacent to the left coronary cusp3,12. Blanke et al defined the tissue in this region of the aortic root as a “locus minoris resistentiae”3. Furthermore, pronounced calcification in the non-coronary valve region could force the frame to overexpand on the left coronary side.
A relatively high proportion of peri-interventional pericardial effusions (19%) was noted in our cohort. One might assume that the annulus rupture was initially uncontained with a small temporary outflow into the pericardial space. Another possible reason for the pericardial effusion could be an injury of the myocardium through a peri-interventional insertion of a temporary pacemaker electrode. Since all ruptures were contained without communication to the pericardium at the time of diagnosis, the exact mechanism remains unclear. However, none of the pericardial effusions resulted in a need for surgical intervention.
The results of our registry show a favourable course of a CR even with continuation of the standard antithrombotic treatment. Therefore, DAPT or antiplatelet monotherapy plus anticoagulation (in patients with an appropriate indication) may be considered in patients with a CR.
Limitations
Only four of the six TAVI centres in our registry performed routine post-TAVI CTA. Due to the clinical characteristics of our patient cohort, the post-TAVI CTA rate was limited, with approximately 60% of the TAVI patients. Therefore, a number of contained ruptures and their corresponding follow-up may have been missed. Furthermore, we cannot exclude deaths due to non-diagnosed uncontained CR immediately after the procedure before performing the post-TAVI CTA. Due to the lack of follow-up CTAs in ten patients (48%), we cannot entirely rule out progression of the CR in some cases. However, none of the CR patients developed symptoms during follow-up and there was no progression in patients with follow-up CTA.
Due to missing data of patients without CR, a calculation of incidences for self-expanding and balloon-expandable or older- and newer-generation THVs was not possible. Furthermore, we cannot exclude an increased risk of overt rupture or other complications, particularly during longer-term follow-up. Despite the limitations, in particular given the low incidence of contained ruptures post TAVI (1.1%), our follow-up data from 21 CR patients can help to clarify the question of the best therapy for this TAVI complication.
Conclusions
The results of our large international registry suggest a favourable course of initially asymptomatic contained ruptures of the aortic annulus after TAVI, supporting a watch-and-wait approach in these patients. Thus, no specific treatment seems to be necessary.
|
Impact on daily practice In view of the fact that further increasing numbers of worldwide TAVI procedures are expected, adequate knowledge in dealing with complications is necessary. Based on the results of our ENCORE registry, a watch-and-wait strategy without specific treatment should be preferred in patients with an asymptomatic contained rupture of the aortic annulus after TAVI. Scientific evidence of a conservative approach is particularly important in TAVI complications, because the initial decision for an interventional treatment of aortic valve stenosis should actually avoid surgery for these patients. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
F-J. Neumann reports institutional grants from Medtronic, Boston, Biotronik and Edwards, and other from The Medicines Company. G. Pache is a consultant for Edwards Lifesciences. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

