Abstract
Background: Although the presence of a thrombus contraindicates left atrial appendage closure procedure (LAAC), a previous study reported the feasibility of the thrombus trapping procedure (TTP) technique to overcome this limitation.
Aims: This study aimed to analyse the short-term outcomes in a series of patients who underwent LAAC using the TTP (TTP-LAAC).
Methods: This retrospective series included patients who underwent TTP-LAAC between January 2018 and May 2020 in 13 European centres. Device choice, pre-interventional work-up and post-discharge antithrombotic therapy regimens were left to the discretion of the operators. The primary endpoint was the 30-day occurrence of stroke, systemic embolism or cardiovascular death.
Results: During the study period, a total of 1,918 patients underwent LAAC. A thrombus was identified in 71 cases but completely disappeared in 24 patients before procedure. TTP-LAAC was finally performed in 53 cases (3%). Thrombi were identified ahead of the actual day of implantation in 47 patients (87%) and were mostly limited in size (50 cases with extension <50% of the LAA surface). The Amplatzer Amulet and WATCHMAN FLX occluders were implanted in 44 and 9 patients, respectively. A single deployment approach was applied in 70% and a cerebral embolic protection system was used in 9% of the patients. The overall success rate was 100%. Small pericardial effusion without tamponade was observed in 6% of the cases. Patients were discharged with 72% under antiplatelet therapy and 10% under short-term oral anticoagulation. The primary endpoint occurred in one patient.
Conclusions: TTP-LAAC might be used in a minority of LAAC procedures but appears to be feasible and safe in the short-term, in select cases.
Introduction
Atrial fibrillation (AF) increases the risk of heart failure, stroke and cardiovascular mortality12. The left atrial appendage (LAA) is the main site for AF-related thrombus formation, which is responsible for stroke3. Percutaneous LAA closure (LAAC) has emerged as a valid alternative therapy for the prevention of systemic embolisms in patients with non-valvular atrial fibrillation and high bleeding risk or definitive contraindication to oral anticoagulation (OAC)456.
LAAC procedure is considered contraindicated in certain situations, including circumstances where an LAA thrombus is identified prior to the intervention. In such cases, the most common option is to provide short-term anticoagulation (either oral or parenteral) in the hopes of offering LAAC once the thrombus has disappeared. This strategy is counterbalanced by the risk of provoking bleeding events. However, some small studies have reported the feasibility of LAAC despite the presence of a pre-existing LAA thrombus (namely: thrombus trapping LAAC [TTP-LAAC])78910. Even if the procedure is associated with higher risk of intraprocedural thromboembolism, it may represent an option for AF patients with LAA thrombus and OAC contraindications or with persistent LAA thrombus despite full OAC.
The Thrombus tRAPing EUropean Registry (TRAPEUR) initiative aimed to create a multicentre registry including patients who underwent TTP-LAAC procedures in different high-volume LAAC centres. The present study sought to investigate the baseline procedural characteristics and outcomes of these patients.
Methods
STUDY DESIGN
The TRAPEUR registry was a multicentre, international, retrospective registry that included patients who underwent a TTP-LAAC procedure in 13 European interventional tertiary cardiology departments that are highly experienced in LAAC procedure. Patients were eligible for inclusion in this registry if they met the following criteria: 1) indication for LAAC procedure (paroxysmal or persistent non-valvular AF with high embolic risk and formal and definitive contraindication to long-term OAC therapy or recurrent ischaemic events in patients with correct OAC therapy), 2) presence of left appendage thrombus on pre-intervention computed-tomography (CT) scan or transoesophageal echocardiography (TEE). The recruitment period for this registry ranged from January 2018 through June 2020.
The study protocol was approved by each local ethics committee and was performed in accordance with the ethical principles stated in the Declaration of Helsinki. All patients gave written informed consent prior to the procedure.
PREPROCEDURAL THROMBUS IDENTIFICATION
The LAA thrombus was identified by a pre-intervention TEE, a computed-tomography CT scan, or at the beginning of the LAAC procedure, according to the local operators’ routine work-up and consensus documents criteria111213 (Figure 1, Moving image 1, Moving image 2). The thrombus was graded as a function of its longitudinal extension: it was considered proximal if it reached the LAA orifice and distal if it was present above the device landing zone. Moreover, LAA thrombus size was quantified as < or > 50% of the LAA total surface area.
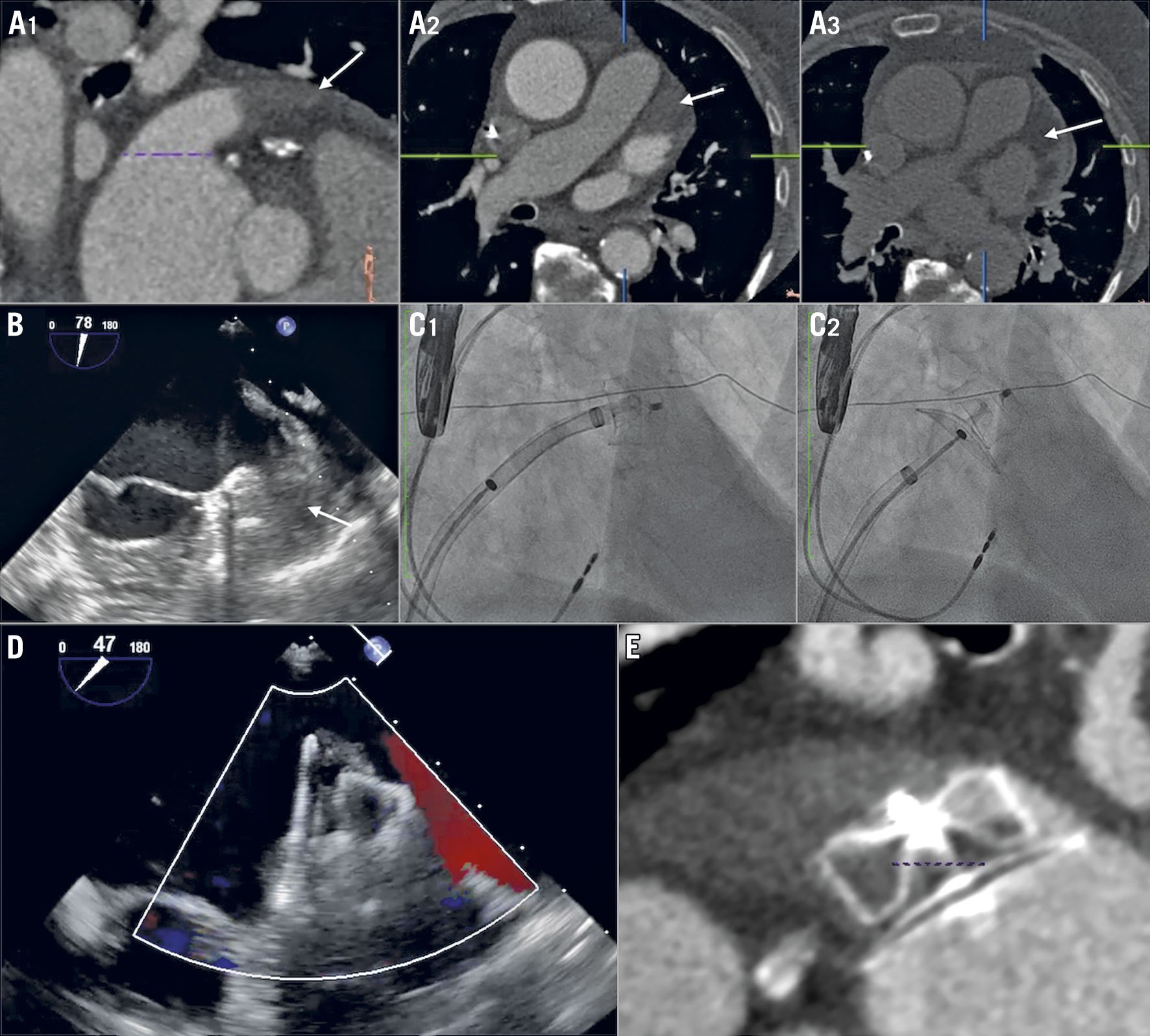
Figure 1. An example of the thrombus trapping procedure. The thrombus (white arrow) was identified within the LAA in a distal position on CT scan at early (A1-A2) and late phase (A3), and was confirmed by periprocedural TEE (B, C1, C2). LAAC was performed using the “single shot” approach with no LAA catheterisation before deployment. The immediate TEE (D) and eight-week results by CT scan (E) were satisfactory. CT: computed tomography; LAA: left atrial appendage; LAAC: left atrial appendage closure; TEE: transoesophageal echocardiography
In some of the cases, the local operators proposed an adjustment of the antithrombotic therapy to reduce the thrombus size prior to the intervention. The options for treatment intensification included low molecular weight heparin (LMWH) injections, short-term oral anticoagulation with vitamin K antagonists (VKA)/direct oral anticoagulants (DOAC) or adjunction of an antiplatelet agent to prior oral anticoagulation.
LEFT ATRIAL APPENDAGE CLOSURE PROCEDURE
The procedures were performed either under conscious sedation with local anaesthesia or under general anaesthesia, using the Amplatzer™ Amulet™ (Abbott Vascular, Santa Clara, CA, USA) or WATCHMAN FLX™ (Boston Scientific, Marlborough, MA, USA) devices12. All patients had an echocardiographic evaluation the day of the procedure using TEE or intracardiac echography (ICE) to establish the presence (or absence) of thrombus, to quantify it (see above) and to identify atrial sludge (Figure 1). The TTP-LAAC procedure differed from conventional LAAC procedures on the following points in order to minimise the risk of thrombus mobilisation (the “no-touch technique”)7: 1) LAA angiography was not performed prior to implantation; 2) pigtail catheters were not inserted in the LAA, 3) the delivery sheath was kept outside the LAA ostium before deployment; and 4) the sheath was cautiously and gently advanced within the LAA once the lobe (Amulet) or the distal part of the prosthesis (WATCHMAN FLX) was partially deployed, in order to deliver the device to the appropriate landing zone. A timed up and go (TUG) test was performed under fluoroscopy by pulling and pushing the delivery cable as appropriate. Coverage of the orifice and residual leaks were evaluated by echocardiography. Although a “single shot” approach was preferred, the device could be cautiously repositioned when necessary. Procedural success was defined as the absence of flow or absence of residual peri-device flow >5 mm assessed by colour Doppler in the LAA. The use of cerebral embolic protection (SENTINEL™; Boston Scientific, Marlborough, MA, USA) was left to the discretion of the operator14. The post-procedural antithrombotic protocol and its duration were left to the discretion of the operator and the local Heart Team.
CLINICAL FOLLOW-UP AND ENDPOINTS
Immediate follow-up was performed in hospital by clinical examination and transthoracic echocardiography (TTE) to rule out device embolisation and pericardial effusion. The follow-up was done by a review of all subsequent clinical records and structured telephone interviews one month after the procedure by an independent research technician. All clinical adverse events were adjudicated by an independent committee as i) related or possibly related to the procedure or ii) not related to the procedure. The adverse events definitions were based on the published LAAC consensus paper15. All the bleeding events were classified according to the definition of the Bleeding Academic Research Consortium (BARC)16.
The primary endpoint of the study was a composite of cardiovascular (CV) mortality, stroke or transient ischaemic attack or peripheral systemic embolism at 30-day follow-up. Secondary endpoints included device embolisation, cardiac tamponade or clinically relevant pericardial effusion, minor or major bleeding, myocardial infarction, and hospitalisation for heart failure.
STATISTICAL ANALYSIS
Quantitative variables were described as median (interquartile range). Categorical variables were described in terms of counts and percentages. All statistical analyses were performed using SPSS 21.0 for MAC (IBM, Armonk, NY, USA).
Results
STUDY POPULATION
Between January 2018 and June 2020, a total of 1,918 consecutive patients underwent LAAC procedure in the 13 centres. Among them, LAA thrombus was identified in 77 patients (4%) including 71 patients before the procedure and 6 patients at the time of the procedure (Figure 2). Short-term anticoagulation was utilised in 24 patients leading to complete resolution of the LAA in all of them and without any major bleeding events; these patients underwent subsequent conventional LAAC. Fifty-three patients (3%) finally underwent a TTP-LAAC procedure (Figure 2, Supplementary Figure 1). In 47 cases, the thrombus was identified before the procedure (direct or deferred TTP-LAAC) and in 6 cases the thrombus was identified the day of the intervention (ad hoc TTP-LAAC). The baseline characteristics of patients treated with TTP-LAAC are given in Table 1. Of the patients, 87% had a prior severe bleeding event (neurological: 34%, gastro-intestinal: 23%, urinary tract: 12%, others: 31%).
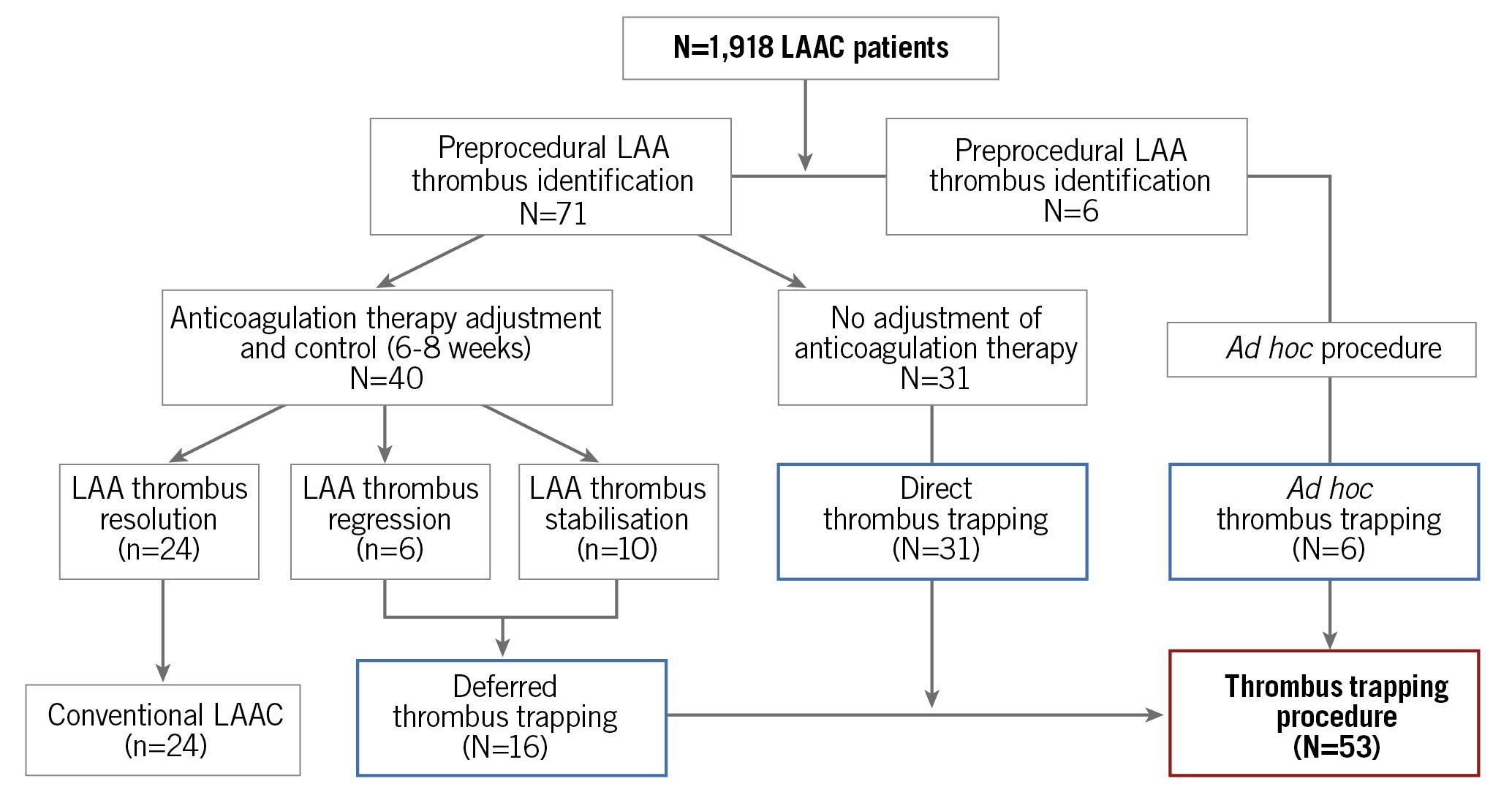
Figure 2. Flow chart of the study population. LAA: left atrial appendage; LAAC: left atrial appendage closure
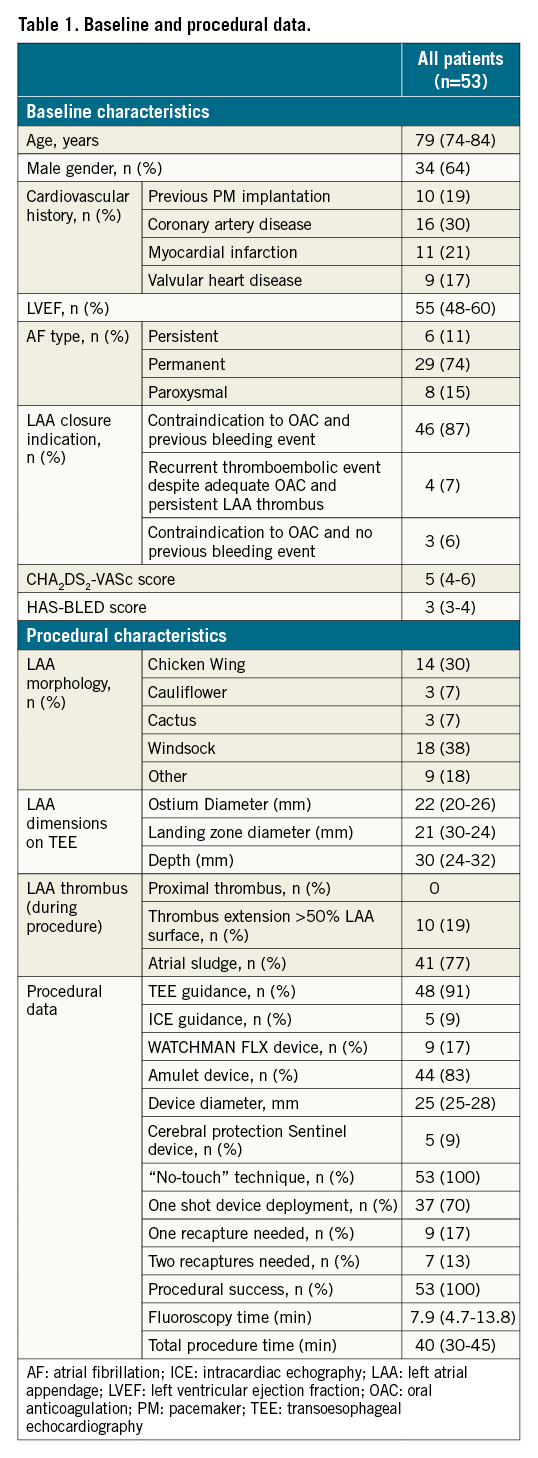
PREPROCEDURAL DATA
The LAA thrombus was identified prior to LAAC by TEE, CT scan or both techniques, respectively, in 7, 18 and 22 patients. The thrombus was proximal in three patients and extended to >50% of the LAA surface in nine patients. An adjustment in antithrombotic therapy was proposed for 16 patients (Figure 2), leading to thrombus regression without disappearance in 6 cases. No recurrent ischaemic event was observed during this interval, but three patients presented significant bleeding events requiring antithrombotic therapy suspension.
PROCEDURAL DATA AND IMMEDIATE OUTCOMES
There was no proximal thrombus at the time of the procedure and there were only three patients with thrombus extension >50% of the LAA surface. The “single-shot” approach (no device recapture) was applied in 70% of the cases. Furthermore, a cerebral protection device was used in 9% of the cases, but no debris was detected in any case after visual inspection with the SENTINEL filter. The Abbott Amulet was the most frequently used device, but there was no significant difference in the characteristics of the patients treated by the two prostheses (data not shown).
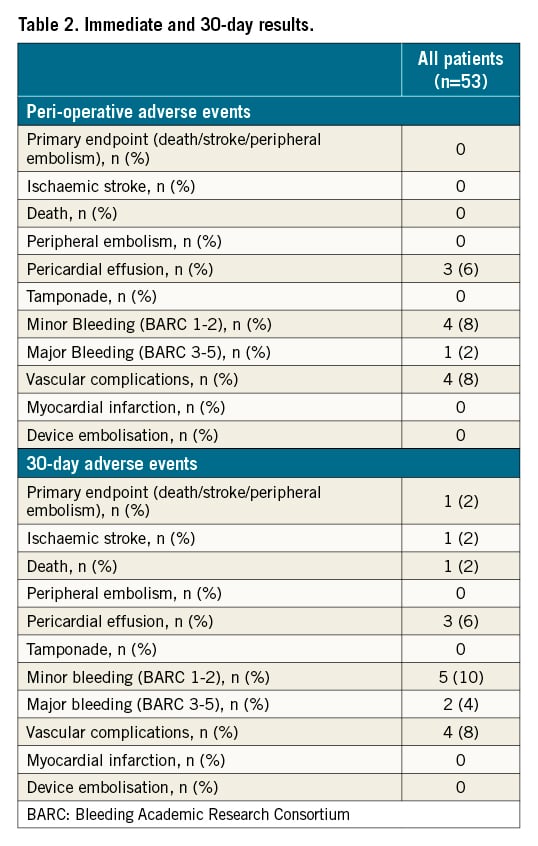
Procedural success was achieved in all patients. There was no periprocedural peripheral embolic event identified. One BARC 3 bleeding (acute anaemia without external haemorrhage requiring blood transfusion) and four minor vascular complications (mild groin haematoma requiring manual compression) were observed. No tamponade was observed but three patients experienced small pericardial effusions without needing drainage (Table 2).
PHARMACOLOGICAL ANTITHROMBOTIC TREATMENT
The evolution of the steps of the antithrombotic strategies is provided in Figure 3. Antiplatelet therapy was the preferred regimen after intervention and 18 patients received dual antiplatelet therapy. Although parenteral or oral anticoagulation was provided in 42% of the patients before LAAC, this percentage decreased to 11% one month after the procedure.
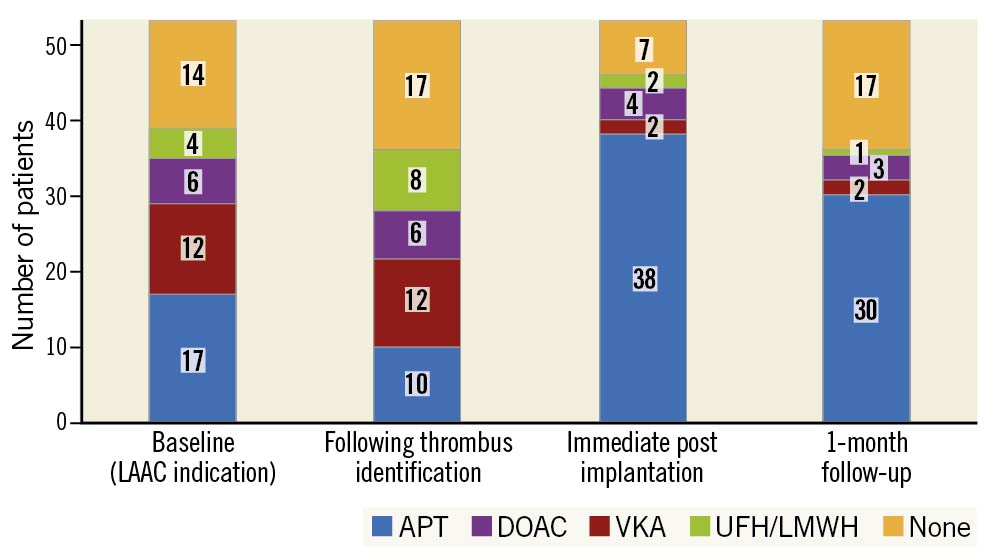
Figure 3. Evolution of antithrombotic therapy during the TTP-LAAC patients’ management (n=53). APT: antiplatelet therapy; DOAC: direct oral anticoagulant; LMWH: low molecular weight heparin; UFH: unfractionated heparin; VKA: vitamin K antagonists
FOLLOW-UP
Thirty-day follow-up was achieved in all patients. The primary endpoint incidence was 2% (95% confidence interval: 0-5.8%) (Table 2). One patient (a direct TTP-LAAC case, without device recapture discharged on dual antiplatelet therapy) suffered from an ischaemic stroke ten days after the initial procedure with subsequent haemorrhagic transformation following intravenous thrombolysis, leading to death. One patient had a re-admission for heart failure during the 30-day follow-up. Moreover, one additional BARC 3 (gastro-intestinal haemorrhage) and one BARC 2 (minor epistaxis) were observed.
Discussion
To the best of our knowledge, the present study provides the first systematic evaluation of TTP-LAAC in a multicentre cohort including nearly 2,000 patients. The main findings can be summarised as follows: 1) LAAC was feasible in patients with pre-existing LAA thrombus; 2) the procedure as carried out limited adverse events with no systemic embolism during periprocedural time, and 3) a preprocedural temporary strategy to increase anticoagulation therapy should be attempted to avoid the presence of a proximal LAA thrombus on the day of the procedure.
The presence of a pre-existing LAA thrombus is a classic contraindication to LAAC intervention, which explains the need of its preprocedural identification by CT scan or TEE. In the current series, thrombus was identified in 4% of all the patients screened for LAAC. The presence of an LAA thrombus is suspected to enhance the risk of procedural stroke by mobilisation or fragmentation during prosthesis implantation and deployment. In cases where an LAA thrombus is identified, the typical management is the intensification of the anticoagulation regimen by increasing the dose, the combination of an antiplatelet agent and OAC or providing a short term parenteral (intravenous or subcutaneous) heparin therapy. However, this strategy can fail to completely clear the appendage or be impossible to apply because of the underlying bleeding risk. In this situation, the TTP-LAAC procedure could be proposed. The technique was initially reported by Jallal et al and differs from the usual intervention by the absence of a pre-closure LAA angiography and avoiding the insertion of a guidewire inside the LAA during the procedure to avoid thrombus manipulation7. TTP-LAAC should ideally be performed with devices that can be implanted without distal LAA sheath manipulation. This explains why the WATCHMAN 2.5™ prosthesis was not implanted in this European series, although a few rare cases have been reported elsewhere810. On the contrary, the newer WATCHMAN FLX device can be deployed without deep intubation in the LAA and thus appears to be more adapted to the TTP-LAAC procedure. In addition, other authors have proposed the use of cerebral protection systems to further reduce the potential risk of thrombus migration14. In our study, this strategy was left to the discretion of the operator and was applied in 9% of our patients (although this rate ranged from 21% to 29% in previous studies810). Several factors could explain this relative underuse, including the lack of availability in some centres, reimbursement issues and the lack of clinical benefit evidence. Hence, previously reported data (and the present series) did not show any differences in periprocedural outcomes in patients with or without SENTINEL use810. Yet this option should be discussed for all TTP-LAAC patients, as it might be applied to patients with the most challenging anatomies (where successive LAAC device recaptures and deployments could be anticipated) or persistent proximal thrombus.
Importantly, our data indicate that the procedure was safe: the 30-day incidence of CV death,/stroke/peripheral embolism or major bleeding events were respectively 2% and 4%. This low rate of major complications is consistent with a recent meta-analysis of 58 patients from 16 publications who underwent TTP-LAAC10. Our study represents the largest cohort of consecutive TTP-LAAC patients to date and allows us to have confidence in the possibility of using this technique. The low rate of periprocedural stroke can be explained by the absence of any proximal thrombus at the time of intervention which was obtained by a careful selection of patients’ CT scans or TEEs before LAAC and/or the modification of an anticoagulation regimen. Although thrombus regression was observed in 75% of the patients in which this latter strategy was proposed, persistent thrombus was identified in 40% of these cases. The LAA thrombus resolution rate in patients with AF but no prior anticoagulation ranges between 80% and 89%, depending on when oral anticoagulants were initiated (VKA or DOAC)171819. However, the benefits of an intensified oral antithrombotic strategy (consisting in increased international normalised ratio (INR) objectives, OAC modification or adjunction of antiplatelet therapy) are poorly documented and might be counterbalanced by the increase of bleeding events. Indeed, Lip and colleagues observed that rivaroxaban treatment initiation in inadequately treated AF patients with LAA thrombus could lead to its complete or partial resolution in 41.5% and 19% of the patients, respectively. There was no change in thrombus size or even increase in 17% and 22.5%, respectively20. The reasons explaining the variable effects of therapy on thrombotic load are not fully understood but might be related to the age, structure and architecture of the clot20. One might also hypothesise that persistent LAA thrombi could also be less fragile and prone to migration during LAAC. In light of these data, a hybrid strategy combining the initial attempt to decrease LAA thrombus size by pharmacologic means followed by deferred or facilitated TTP-LAAC might represent a sound approach, especially in patients with proximal clots (Figure 4). In addition, we observed small pericardial effusion in 6% of the cases. This rate is higher than expected compared to “classical” LAAC procedures6, which can be explained by differences in procedural strategy (not using pigtail catheters, no LAA injection, etc.) and the small sample size. However, none of these effusions led to tamponade or specific therapy and thus could be considered as minor complications.
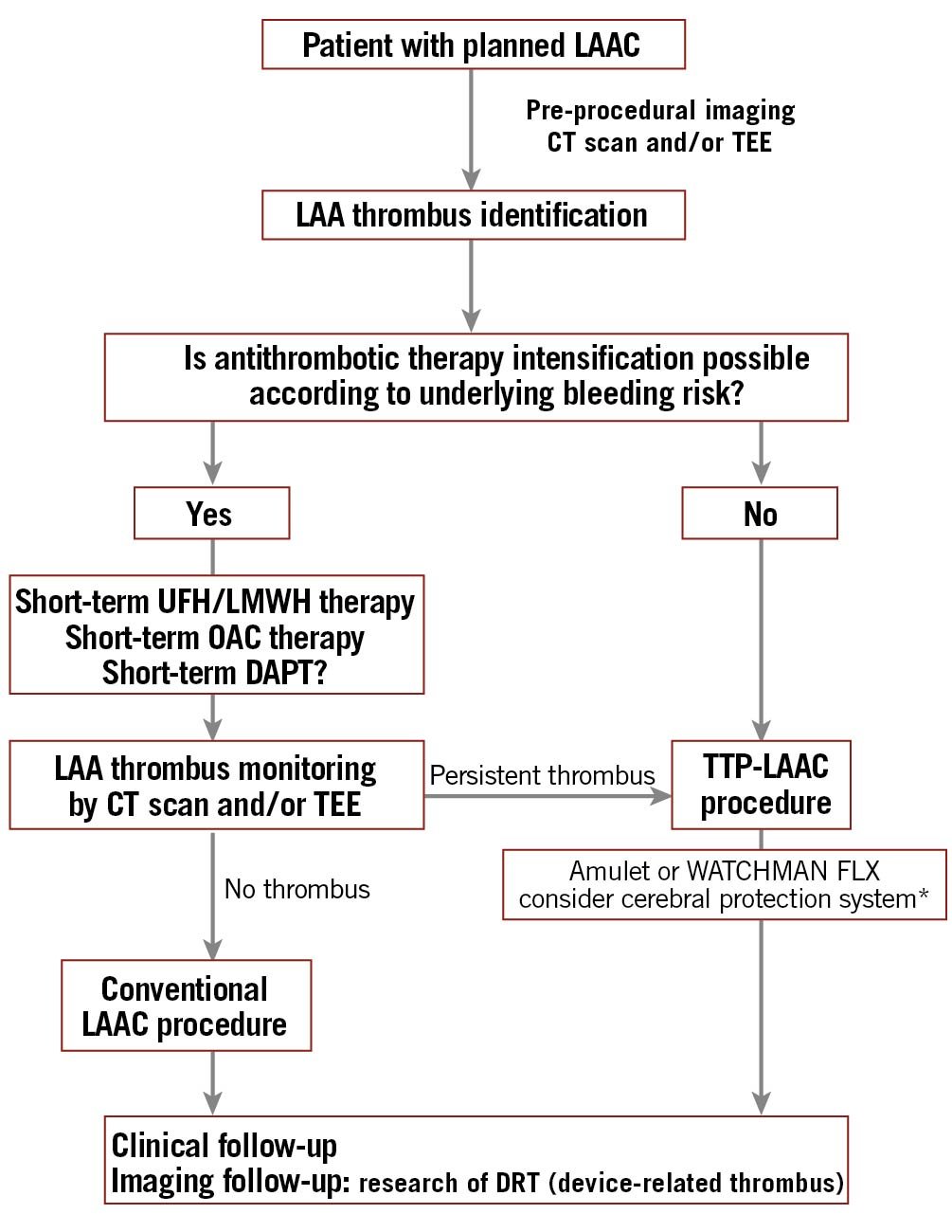
Figure 4. Proposed management algorithm for patients with planned LAAC and thrombus identification. * Hypothesis generated, without strong evidence supporting it. CT: computed tomography; DAPT: double antiplatelet therapy; DRT: device related thrombus; LAA: left atrial appendage; LAAC: left atrial appendage closure; LMWH: low molecular weight heparin; OAC: oral anticoagulation; TEE: transoesophageal echocardiography; TTP: thrombus trapping procedure; UFH: unfractionated heparin
Although attractive, the TTP-LAAC intervention should remain an exceptional procedure and only be proposed in very select cases and exclusively with LAA distal thrombus, following discussion with the Heart Team. Hence, in this series TTP-LAAC only represented 3% of the total number of LAAC cases performed in the centres during the inclusion period, but the success rate was 100% (Central illustration).

Central illustration. Key baseline and procedural characteristics and main results in the TRAPEUR registry. LAA: left atrial appendage; LAAC: left atrial appendage closure
Limitations
Although our study represents the largest cohort of TTP-LAAC patients to date, the sample size remained limited due to the low number of patients eligible for this procedure. Moreover, the preprocedural and procedural management were not homogeneous among the 13 centres and reflect local European practices. The diagnosis of thrombus was supported by CT scan according to the latest recommendations (late phase imaging) in most cases (40/53 cases)13. In case of sole TEE use, the isoproterenol infusion was left to the discretion of the operators, but other methods (intra-procedural LAA heparin or contrast injection) were not applied in order to avoid LAA intubation. There was no centralised review of the imaging and procedural data.
In addition, the TRAPEUR operators performed procedures in high volume LAAC centres and thus, the results might not be the same for less experienced operators. Moreover, most patients were implanted with the Amulet device and the translation of the results to other occluders is questionable. Furthermore, the one-month clinical follow-up was short. This design was chosen to evaluate the procedure success rate and periprocedural complications, which were the study’s primary aims. However, a longer follow-up would be needed to further assess subsequent outcomes and the risk of late complications, including the risk of device-related thrombus (DRT). There was also no evaluation of potential postprocedural silent brain lesions by systematic MRI. Finally, the antithrombotic strategy following the implantation was not standardised and the best antithrombotic strategy in this population still needs to be assessed (see above).
Conclusions
LAA closure appears to be feasible and safe in selected AF patients with persistent LAA thrombus. A trial of an intensified anticoagulation regimen, no-touch deployment technique, cerebral embolic protection, and detailed review of the risks and benefits need to be considered in these high-risk cases.
Impact on daily practice
Although in situ thrombus represents a classic contraindication to the left atrial appendage closure procedures, this series shows that LAA closure by TTP is feasible and safe in some select cases. The procedure should be modified to avoid thrombus mobilisation, including the absence of guidewire or sheath manipulation in the distal LAA and no appendage opacification. Further studies are needed to establish the indications of thrombus trapping indications in LAAC and the optimal antithrombotic drug management supporting the procedure.
Conflict of interest statement
F.A. Sebag reports consulting fees from Abbott and Biosense Webster. P. Garot is a minor shareholder in the Cardiovascular European Research Center (CERC). O. De Backer reports institutional research grants and consulting fees for Abbott and Boston Scientific. D. Hildick-Smith is a proctor and on the advisory board of Abbott and Boston Scientific. G. Moubarak reports consulting fees for Abbott. G. Ducrocq reports speaker and/or consulting fees for Amgen, Astra Zeneca, Bayer, BMS, Janssen, Sanofi and Terumo. He has also served as a proctor for Boston Scientific. R. Eschalier reports consulting fees from Boston Scientific. A. Aminian reports proctoring and consulting fees for Abbott and Boston Scientific. N. Lellouche reports consulting fees for Abbott and Boston Scientific. L. Räber reports research grants to his institution from Abbott, Boston Scientific, Biotronik, Medis, Heartflow, Sanofi and Regeneron as well as speaker/consultation fees for Abbott, Amgen, AstraZeneca, Canon, Occlutech, Sanofi and Vifor. N. Amabile reports proctoring for Abbott and Boston Scientific, reports consulting/lecturing fees from Abbott, Boston Scientific and Shockwave and reports research grants to his institution from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. An example of large LAA thrombus identified by TEE.
Moving image 2. Another example of LAA thrombus identified by TEE.

