Abstract
Aims: The elastic behaviour (acute recoil) of a valve prosthesis stent following transcatheter aortic valve implantation (TAVI) is unknown. This study sought to determine the occurrence, severity, predictive factors and haemodynamic consequences of acute recoil following TAVI.
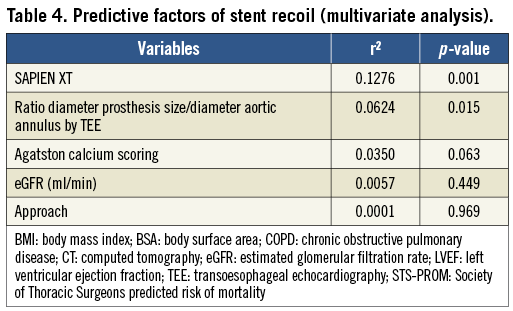
Methods and results: A prospective angiographic analysis of the stent frame dimensions in 111 consecutive patients who underwent TAVI with a balloon-expandable valve (36 Edwards SAPIEN; 75 SAPIEN XT) was performed. Acute recoil was defined as the difference between minimal lumen diameter (MLD) at full balloon expansion and immediately after balloon deflation. MLD during balloon inflation was significantly larger than MLD after balloon deflation (23.40±2.31 mm vs. 22.29±2.21 mm, p<0.001), which represented an absolute and percent decrease in stent dimension of 1.10±0.40 mm and 4.70±1.76%, respectively. In the multivariate analysis, the predictors of larger recoil were a higher prosthesis/annulus ratio (r²=0.0624, p=0.015) and the SAPIEN XT prosthesis (r²=0.1276, p=0.001). No significant changes in haemodynamic performance were observed at discharge and follow-up in patients with larger recoil.
Conclusions: TAVI with a balloon-expandable valve was systematically associated with a certain degree of valve stent recoil after balloon deflation. A higher degree of valve oversizing and the SAPIEN XT prosthesis predicted a larger degree of stent recoil.
Introduction
Elastic recoil of stents is a well-known phenomenon in the coronary field, and has angiographic consequences for the potentially achievable luminal diameter and clinical implications after successful coronary angioplasty1-4. Intrinsic (stent design and materials) and extrinsic (compressive arterial forces) factors can contribute to elastic recoil after balloon deflation and, therefore, to the final stent deployment diameters5,6. Transcatheter heart valves (THV) mounted on a stent frame are increasingly being used as an alternative to aortic valve surgery in high-risk patients with severe aortic stenosis7. To obtain proper anchoring, the stent is systematically oversized by 2 to 5 mm with respect to the diameter of the aortic annulus, so the aortic annulus might exert a compression pressure on the bioprosthesis and a decrease in the stent diameter can be expected after balloon deflation, in the case of balloon-expandable valves. However, no data exist on the elastic behaviour of the stent frame of the balloon-expandable THVs during balloon deflation and on the factors associated with this phenomenon. Moreover, successful deployment and function in TAVI is heavily reliant on the tissue-stent interaction and significant recoil could theoretically have a bearing on valve gradients and regurgitation. The purpose of this study, therefore, was to evaluate the occurrence and predictive factors of immediate recoil after TAVI with a balloon-expandable valve, and its acute and midterm haemodynamic consequences.
Methods
STUDY POPULATION AND TAVI PROCEDURES
A total of 111 consecutive patients diagnosed with symptomatic severe aortic stenosis who underwent TAVI with a balloon-expandable valve at our institution were included. Selection of transfemoral versus transapical or transaortic approaches was based on the appropriateness of the iliofemoral axis. All procedures were performed under general anaesthesia and guided by fluoroscopy/angiography and transoesophageal echocardiography (TEE). The size of the valve prosthesis was selected on the basis of aortic annulus measurements obtained by TEE (first 25 patients) or by a combination of TEE and computed tomography (CT) (last 86 patients). Valve sizes of 23, 26, and 29 mm were selected for aortic annuli between 18 and 21 mm, 22 and 24 mm, and 25 and 27 mm, respectively. Full balloon inflation lasted for at least three seconds during valve implantation. Balloon post-dilation adding 0.5 to 1 ml of saline to the same balloon used for valve implantation was performed in case of significant paravalvular aortic regurgitation (grade ≥2). Baseline and periprocedural data were prospectively collected in a dedicated database. All procedures were performed under a compassionate clinical use programme approved by Health Canada (Ottawa, Ontario, Canada), and all patients provided written informed consent for the procedures.
ANGIOGRAPHIC ANALYSIS
The valve deployment process was specifically recorded in order to select and analyse two angiographic images for the assessment of acute stent recoil, one at the end of full balloon expansion and the other immediately following complete balloon deflation. The time interval between these two images was less than one minute. Both images were analysed in the same angiographic projection which displayed the three aortic valve sinuses in line to minimise the possibility of foreshortening during measurement. Accurate calibration of the system was performed using the two radiopaque markers of the balloon. Stent dimensions were assessed at three different levels of the stent frame: a) upper (aortic) level, b) mid level, and c) lower (ventricular) level (Figure 1). The minimum measurement obtained was considered the stent minimal lumen diameter (MLD). The mean of the three measurements was considered the stent mean diameter.
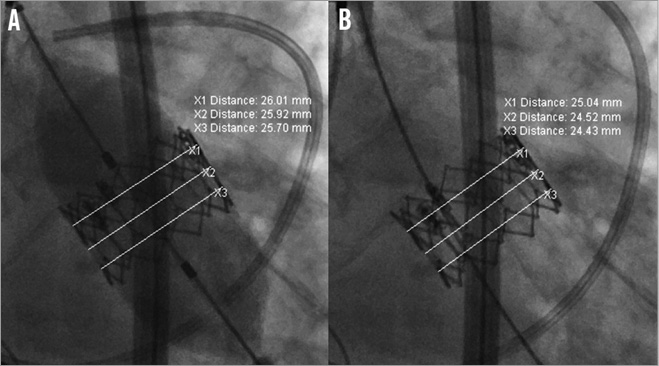
Figure 1. Acute stent recoil assessment. Stent diameter measurements (aortic level –X1, mid level –X2, ventricular level –X3) of a 26 mm Edwards SAPIEN XT on angiography at full balloon expansion (A) and immediately after balloon deflation (B). In this particular case, the minimal lumen diameters at full balloon expansion and following balloon deflation are 25.70 mm and 24.43 mm, respectively. This corresponds to acute absolute and percent stent recoil of 1.27 mm and 4.94%, respectively.
Acute stent recoil was calculated as the difference between MLD of the stent at full balloon expansion (X) and MLD of the stent after balloon deflation (Y). Whereas absolute acute stent recoil was calculated as the difference between X-Y, relative acute stent recoil was defined as (X-Y)/X and expressed as a percentage (Figure 1). In the case of balloon post-dilation, the same formula was used to calculate acute stent recoil. The mean absolute increase in stent MLD was defined as the difference between MLD before and after balloon post-dilation. The angiographic measurements were performed immediately after the procedure by one investigator unaware of the clinical data. The intraobserver agreement for stent measurements was assessed on a subsample of 20 patients at least one month after the initial evaluation and the assessors were blinded to the previous results. The mean standard deviation (SD) for intraobserver differences in repeated measurements was 0.08 mm for acute absolute recoil and 0.35% for relative recoil. A difference of >3 times the mean SD for differences in repeated measurements was considered significant. Consequently, significant absolute recoil and relative acute recoil were defined as a decrease of ≥0.24 mm and ≥1.06% in MLD, respectively, after balloon deflation.
ECHOCARDIOGRAPHY EVALUATION
Transthoracic echocardiography studies were systematically performed at baseline, at hospital discharge and at six months. All exams were analysed by experienced technicians blinded to clinical data and supervised by a cardiologist at the Echo Core Lab of the Quebec Heart & Lung Institute. Transvalvular gradients and valve effective orifice area measurements were performed as previously described8. The severity of AR was evaluated using the multiparametric approach proposed in the American Society of Echocardiography/European Association of Echocardiography guidelines9,10.
COMPUTED TOMOGRAPHY: ANALYSIS OF VALVE CALCIFICATION AND SIZING
A total of 86 patients underwent thoracic CT before the procedure. The CT images of the aortic valve were analysed offline by experienced cardiologists and radiologists blinded to clinical data. Three-dimensional multiplanar reconstruction was performed to examine the aortic valve in-plane (2 mm slice thickness, two to five slices per valve for full coverage) and to measure precisely leaflet calcifications defined as pixels >130 Hounsfield units (TeraRecon, San Mateo, CA, USA) as previously described by Agatston et al11. In addition, annulus sizing was evaluated in a double oblique transverse imaging orthogonal to the aortic root at the level of the basal ring obtaining the maximal and minimal annulus diameters and annulus area12.
STATISTICAL ANALYSIS
Qualitative variables were expressed as percentages and continuous variables as mean values with their SD or as median values with their 25% to 75% interquartile range, according to data distribution performed by the Kolmogorov-Smirnov test. Comparison of numerical variables was performed using the Student’s t-test or Wilcoxon rank test depending on variable distribution, and the chi-square test or Fisher’s exact test was used to compare qualitative variables. The variables associated with a higher acute relative recoil with a p-value <0.10 on univariate analysis were entered in a multivariate linear regression model to determine the independent predictors of relative recoil. Linear regression analyses were performed to test the correlation between the degree of recoil and valve haemodynamics. The results were considered significant with p-values <0.05. All analyses were conducted using the statistical package SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The clinical, echocardiographic, and procedural characteristics of the study population are shown in Table 1.
ACUTE STENT RECOIL RESULTS
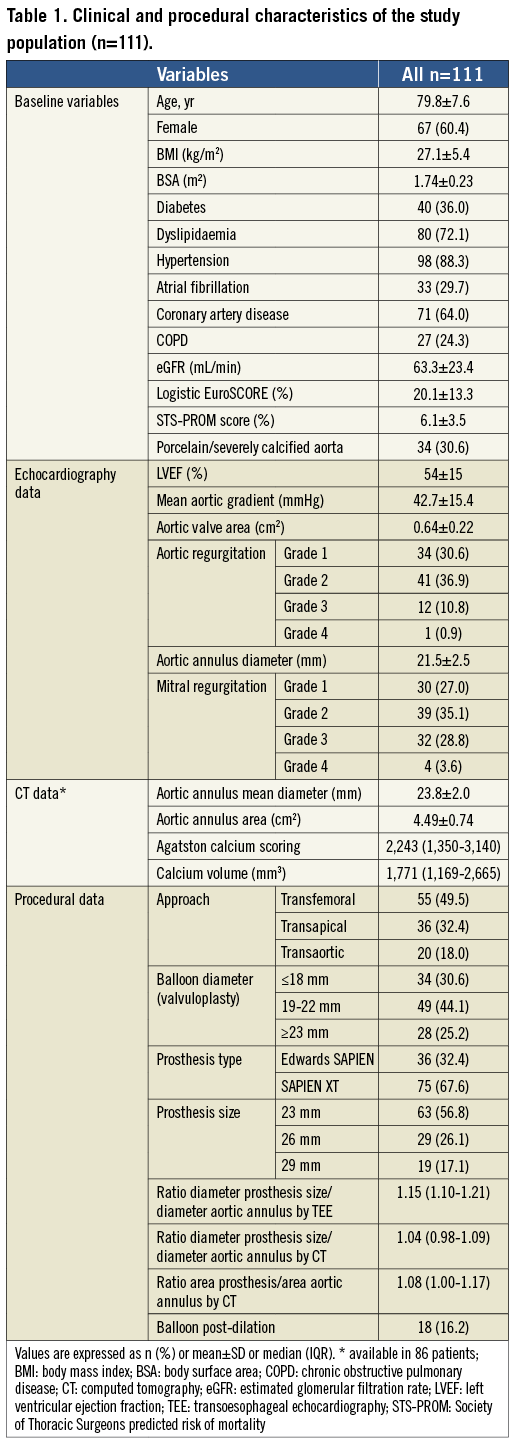
Measurements of the stent-frame dimensions are shown in Table 2. Stent prosthesis diameters at full balloon expansion were significantly higher when compared to stent diameters after balloon deflation (p=0.001 for all analyses) (Figure 2). Final stent diameters at the upper (aortic) stent level tended to be larger than at the mid level (p=0.102) and at the lower (ventricular) level (p=0.051). The MLD during balloon expansion and deflation was at the ventricular level in 53% and 57% of the cases, respectively, and at the mid level in 43% and 39% of the cases. Some degree of absolute (MLD decrease ≥0.24 mm) and relative (MLD decrease ≥1.06%) stent recoil was observed in 110 (99.1%) patients. The mean absolute decrease in stent MLD after balloon deflation was 1.10±0.42 mm, which represented a mean percent decrease of 4.70±1.76% (Figure 2). The absolute and percent decrease in stent dimensions was similar in the three levels (p=0.699 for absolute recoil; p=0.422 for relative recoil). Similar results were obtained when mean stent diameters were used for analysis (Table 2 and Figure 2). Measurements and recoil of the stent according to valve size are shown in Table 3. The final diameters of the 23, 26 and 29 mm valves were 20.68±0.88 mm, 23.57±1.08 mm and 25.70±1.27 mm, respectively, which represented a relative underexpansion of 10.1%, 9.3% and 11.4%, respectively. Acute absolute recoil was higher (p=0.020) in the 29 mm valve (1.29±0.35 mm) compared to the 23 mm valve (1.04±0.40 mm), but relative acute recoil was similar for the two valve sizes (4.76±1.25% for the 29 mm valve; 4.78±1.83% for the 23 mm valve, p=0.954) (Figure 3).
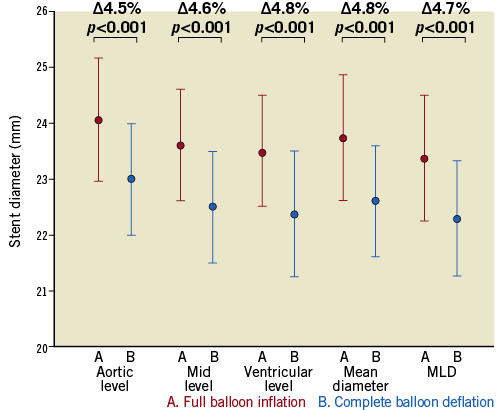
Figure 2. Stent dimensions of the transcatheter heart valve. Mean stent diameter at three levels (aortic, mid and ventricular), mean and minimal lumen diameter at full balloon inflation (A) and immediately after complete balloon deflation (B).
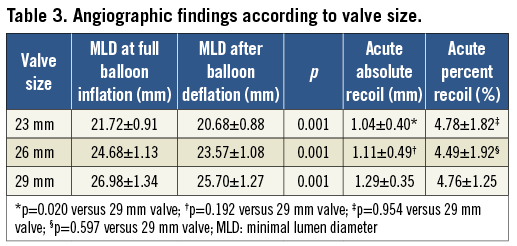
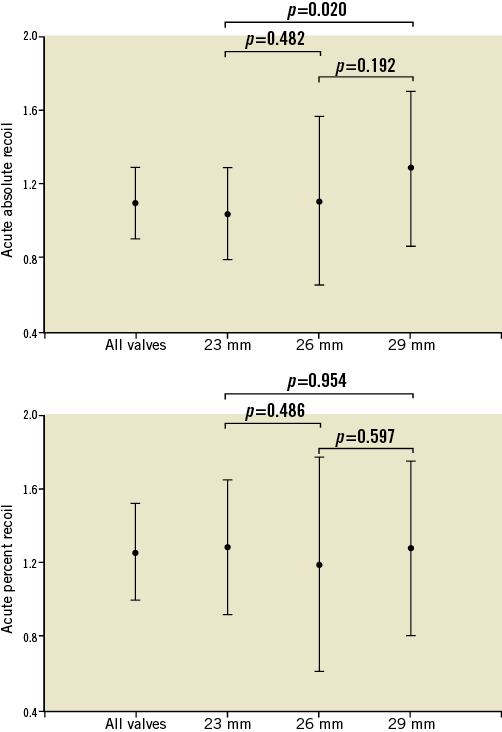
Figure 3. Acute absolute and relative stent recoil. Acute absolute (mm) and relative (%) stent recoil following transcatheter aortic valve implantation, according to valve size.
Acute recoil was greater in the lower annulus range for the 23 mm valve (annulus of ≤19.5 mm: 5.34±1.83%; annulus of >19.5 mm: 4.37±1.73%, p=0.036), and tended to be greater in the lower annulus range for the 26 mm (annulus of ≤23 mm: 4.96±1.75%; annulus of >23 mm: 3.60±2.00%, p=0.069) and 29 mm (annulus of ≤26 mm: 5.27±1.17%; annulus of >26 mm: 4.52±1.25%, p=0.236) valves. Balloon post-dilation was performed in 18 (16%) patients. Recoil with balloon post-dilation was significantly lower compared to the valve implantation process (3.5±1.52% vs. 4.70±1.76%, p=0.003). The mean absolute increase in stent MLD after balloon post-dilation was 0.81 mm (95% CI: 0.64 to 0.99 mm, p<0.001), which represented a mean percent increase of 3.6% (95% CI: 2.9% to 4.5%).
PREDICTORS OF ACUTE PERCENT STENT RECOIL
The degree of recoil according to baseline and procedural characteristics of the entire study population is shown in Figure 4. Poor kidney function, higher degree of valve calcification, transfemoral approach, the Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA) and greater degree of oversizing (valve/annulus ratio) were associated with higher relative recoil. In the multivariate analysis, the SAPIEN XT valve (r2=0.1276, p=0.001) and a higher valve/annulus ratio determined by TEE (r2=0.0624, p=0.015) were the independent predictors of a larger degree of stent recoil (Table 4). Measurements of the balloon at full expansion were similar between the Edwards SAPIEN and SAPIEN XT prostheses (p=0.978 for the 23 mm valves; p=0.526 for the 26 mm valves).
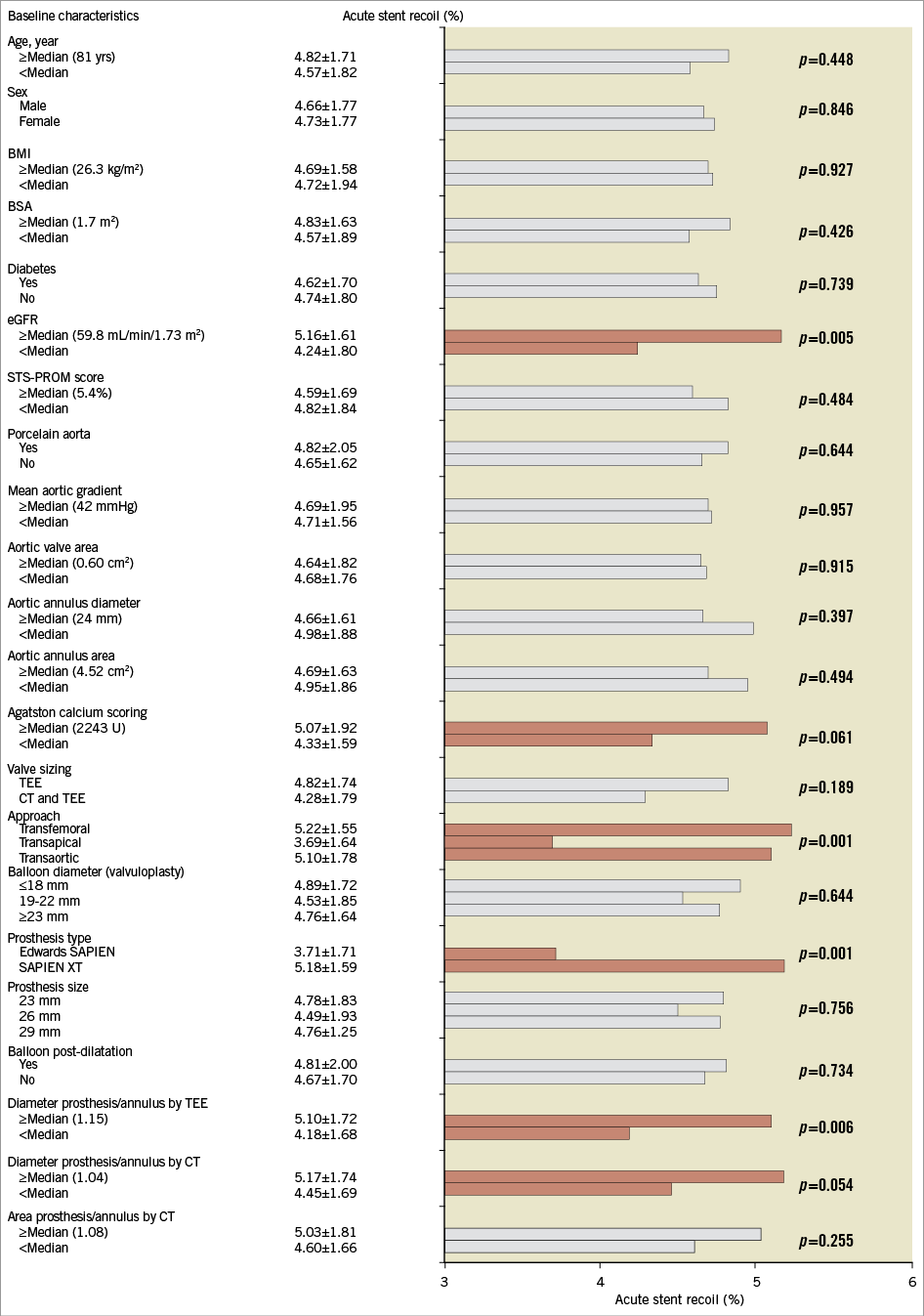
Figure 4. Acute relative stent recoil following TAVI according to clinical, echocardiographic, computed tomography and procedural variables. BMI: body mass index; BSA: body surface area; CT: computed tomography; eGFR: estimated glomerular filtration rate; TEE: transoesophageal echocardiography; STS-PROM: Society of Thoracic Surgeons predicted risk of mortality
VALVE HAEMODYNAMICS
The degree of recoil did not correlate with the mean residual gradient (r=–0.003, p=0.979) or aortic valve area (r=0.103, p=0.378) at hospital discharge (Figure 5). The relative recoil was 4.93±1.75% and 4.63±1.77%, respectively, in patients with (n=26, 23.4%) and without (n=85, 76.6%) residual aortic regurgitation ≥2 (p=0.44). At six-month follow-up, valve haemodynamics remained stable and no correlation was found with the degree of acute recoil (Figure 5).
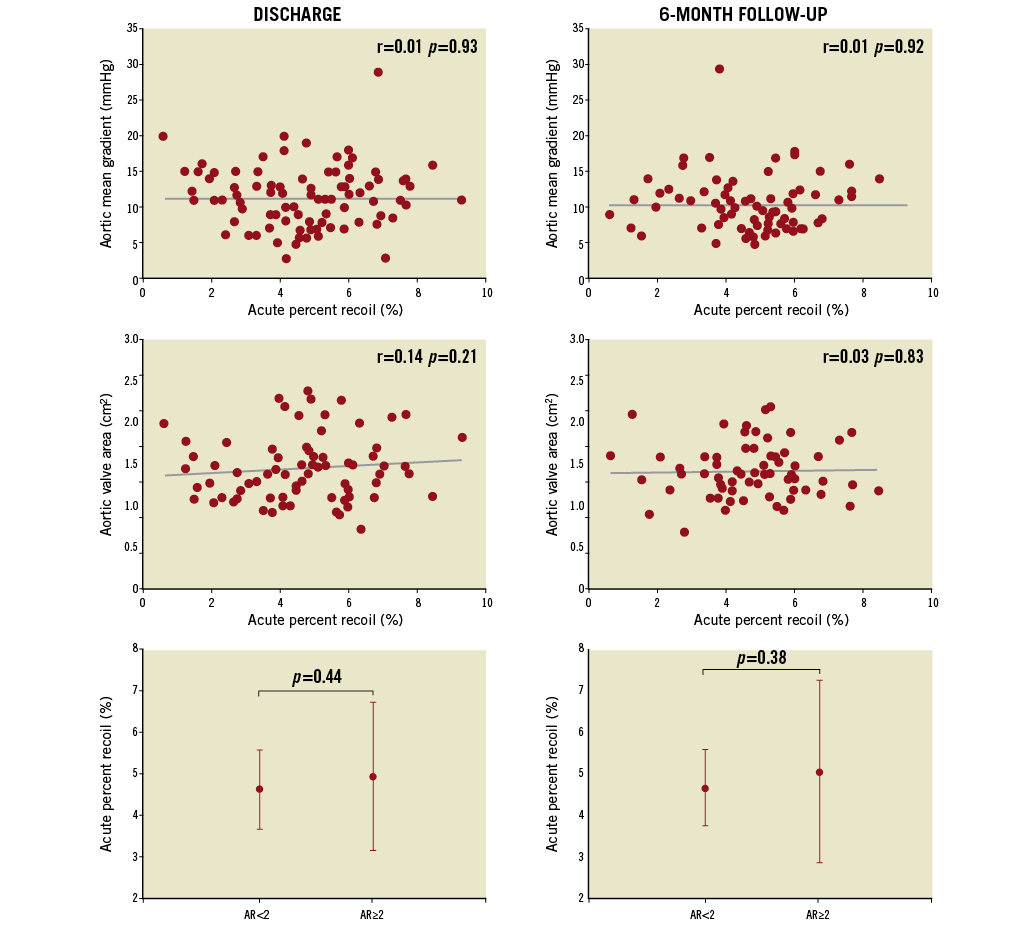
Figure 5. Acute relative recoil and valve haemodynamics. Correlations between valve haemodynamics (mean transvalvular gradient, aortic valve area and residual aortic regurgitation) and the degree of relative stent recoil at hospital discharge and six-month follow-up.
Discussion
The present study showed that balloon-expandable THVs almost systematically exhibited a certain degree of stent recoil after balloon deflation (mean values for absolute and relative recoil of 1.1 mm and 4.7%, respectively). The diameter of the stent at the aortic level tended to be larger than the diameters at the mid or ventricular level, but the absolute and percent decreases in stent diameters were similar in the three levels. The degree of valve oversizing and the use of a SAPIEN XT (vs. Edwards SAPIEN) valve predicted a greater degree of stent recoil. Valve haemodynamics were not influenced by the degree of stent recoil of the THV.
FREQUENCY AND DEGREE OF RECOIL
Very few data are available on the elastic behaviour of the stent frame of THVs during the implantation process. Schultz et al13 examined the stent frame dimensions and apposition to the native anatomy by CT after TAVI with the self-expandable CoreValve system (Medtronic, St. Paul, MN, USA). Incomplete expansion was found in all patients and none of the stent frames reached the expected nominal dimensions, with a relative undersize of about 24%. Also, Jilaihawi et al14 described the occurrence of incomplete stent expansion in 55% of patients who had undergone TAVI with a self-expandable valve. Little is known about the final angiographic dimensions of the balloon-expandable THVs. The present study showed a systematic underexpansion of the balloon-expandable THVs, with a relative undersize of about 10%. This degree of underexpansion seems to be lower than that reported for self-expandable valves13: this may be related to the fact that radial forces obtained with self-expandable valves are lower than those obtained with balloon-expandable valves15 and to differences in the degree of valve oversizing between valve types. These data could have implications for selecting the valve size in order to minimise residual paravalvular regurgitation. Taking this a step further, the present study was the first to assess the dynamic performance of a balloon-expandable valve during the implantation process, showing that some decrease in stent dimensions occurs almost systematically after balloon deflation and contributes to the failure to achieve predicted final stent dimensions. In previous coronary studies, the percentage of stent recoil was about 6% (ranging from 3 to 18%) as measured by quantitative coronary angiography2,16-18, which is close to the degree of recoil observed in the present study. Importantly, the degree of acute relative recoil was comparable across the three levels of the valve stent frame, highlighting the fact that the elastic behaviour is homogenous in the entire prosthesis. However, the maximal stent diameter was significantly larger than the MLD, and the final diameter at the upper (aortic) stent level was slightly larger compared to the other two –mid and ventricular– stent segments (Table 2). This mild distortion of the valve stent as well as valve underexpansion might be related to the structure of the frame and the skirt attached near the ventricular segment (intrinsic factors), and differences in external compression forces that the aortic outflow exerts on the prosthesis and the amount and distribution of calcium within the valve and annulus (extrinsic factors). Interestingly, the 29 mm valve exhibited a higher degree of absolute recoil than the 23 mm prosthesis, but the relative recoil was similar among valve sizes. This is in accordance with coronary stent studies, where the relative recoil was not influenced by the reference vessel and stent diameters16.
PREDICTORS OF RECOIL
No studies to date have specifically evaluated the predictors of recoil during TAVI procedures. The results of this study showed that immediate recoil was independent of baseline clinical characteristics and was related to intrinsic factors (prosthesis type) and to extrinsic factors (anatomical elements).
In coronary studies, many properties of the stent such as rigidity, elastic recoil, and visibility have been shown to be affected by stent design and materials. Coil tube and cobalt-chromium stents have been related to a greater degree of recoil19,20. The first two generations of the Edwards valves (Cribier-Edwards and Edwards SAPIEN) were mounted in a stainless steel stent, and the stent frame of the third-generation Edwards valve, the SAPIEN XT valve, consists of a cobalt-chromium stent containing a more open cell design that enables a lower crimped profile. Cobalt-chromium stents provide higher flexibility and thinner struts, but have also been associated with greater acute recoil than stainless steel stents20,21. In addition, the fact that the metallic surface is smaller with SAPIEN XT may be responsible for the greater recoil observed with this THV. Further studies are needed to quantify detailed material properties and the interaction with the tissues in the annulus region.
The present study shows that the degree of oversizing is also an important factor in determining the degree of recoil after the implantation of a balloon-expandable valve. Several studies in the coronary field have shown that a higher balloon/vessel ratio leads to a greater degree of elastic recoil3,22. Mummert et al23 reported, in an experimental model, that aortic root compression forces were dependent on the amount of THV oversizing. Consistent with these results, Clavel et al8 showed that the main factor limiting the full expansion of the valve, and consequently the final aortic valve area, was the size of the native aortic annulus. Each THV is used for a wide aortic annulus size range, and the compression forces would be greater if a 23 mm valve was implanted in an 18 mm rather than in a 21 mm annulus. Thus, accurate measurements of the aortic annulus with multiple imaging techniques and an adequate range of prosthesis sizes are necessary to obtain the optimal valve/annulus ratio and to minimise both the recoil phenomenon and the rate of complications such as aortic annulus rupture, paravalvular leakage and device migration.
VALVE HAEMODYNAMICS
The degree of recoil of the THV was not related to valve function, unlike the coronary field, where the final lumen diameter is an important factor in angiographic and clinical outcomes. While a significant decrease in stent dimensions might translate into a lower valve area and a higher rate of significant paravalvular aortic regurgitation, the present study showed that mean gradient, aortic valve area and the degree and severity of aortic regurgitation at hospital discharge and six-month follow-up were similar in patients with larger recoil. Thus, this study suggests that recoil would probably not have an effect on valve dysfunction. Future studies including a larger number of patients will have to evaluate further the potential clinical impact of this effect on valve haemodynamics. As reported above, the frequency of aortic regurgitation ≥grade 2 was 23.4% and remained stable at six-month follow-up. Interestingly, patients with larger recoil had a tendency to a greater Agatston calcium score in the analysis, and it is well known that the amount of valve calcification is an important predictor of significant aortic regurgitation with self-expandable and balloon-expandable valves24-26. The impact of valve calcification on the degree of recoil and paravalvular regurgitation needs to be addressed in future studies.
Limitations
Although stent dimensions were measured in an angiographic projection with the three aortic valve sinuses in line to reduce foreshortening, 3-D images would give a more accurate idea of the real stent dimensions. We assumed a uniform and circumferential expansion of the balloon at full inflation, but we cannot rule out an eccentric shape of the valve. However, given that the degree of recoil is expressed as a percentage, the possible error in our analysis is probably negligible. Annulus size and calcium data measured by CT were not available in 22% of the patients, and this might have led to an underestimation of the importance of these variables as predictors of recoil. Finally, the sample size was limited and the study might be underpowered to detect an impact of acute recoil on valve haemodynamics.
Conclusions
In conclusion, a certain degree of acute stent recoil of balloon-expandable THVs occurred nearly systematically after balloon deflation. The relative retraction in stent dimensions was homogenous across stent valve levels and sizes. The degree of annulus oversizing and the SAPIEN XT device were the predictive factors of a higher degree of stent recoil. The presence and severity of acute recoil were not associated with any deleterious effect on valve function acutely and at midterm follow-up. Future studies with a larger number of patients and longer follow-up are needed to determine further the potential clinical impact of stent recoil associated with balloon-expandable THVs.
Acknowledgements
The authors would like to thank the team of radiology technicians from the Quebec Heart & Lung Institute for their constant help in acquiring the images.
Funding
Luis Nombela-Franco received funding via a research grant from the Fundación Mutua Madrileña (Spain). H. B. Ribeiro is supported by a research PhD grant from CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil.
Conflict of interest statement
J. Rodés-Cabau is a consultant for Edwards Lifesciences Inc. and St. Jude Medical. R. DeLarochellière is a consultant for St. Jude Medical, and E. Dumont is a consultant for Edwards Lifesciences Inc. The other authors have no conflicts of interest to declare.

