
Abstract
Aims: The aim of this study was to assess in vitro the diagnostic accuracy of computed tomography angiography (CTA) for the evaluation of complex coronary lesions.
Methods and results: Five Plexiglas phantoms with three bifurcation lesions each were designed to mimic the anatomic variations and fractal phenomena of the coronary tree. In addition, luminal stenoses were scaled up with increases of 10% from 40% to 80%, corresponding to luminal areas ranging from 3.0 mm2 to 0.22 mm2. Third-generation dual-source computed tomography was used. Automated quantitative CTA analysis was performed according to the bifurcation segment model. The primary objective was to determine the diagnostic accuracy of quantitative CTA in assessing bifurcation lesions with the phantoms as a reference. The accuracy of CTA for the assessment of minimal luminal diameter was –0.07 mm (limits of agreement –0.75 to 0.61), for reference vessel diameter 0.19 mm (limits of agreement –0.25 to 0.63) and diameter stenosis 8.2% (limits of agreement –13.2 to 29.5) with no difference regarding the location within the bifurcation (i.e., proximal and distal main vessel and side branch). In stenosis with minimal luminal diameter ≥1 mm, CTA overestimated the lesion severity (bias 0.19 mm, limits of agreement –0.09 to 0.47), whereas in lesions with severe stenosis and minimal luminal diameter ≤1 mm, CTA underestimated the lesion severity (bias –0.48 mm, limits of agreement –0.55 to –0.41). CTA was able to identify the contrast-filled lumen in all degrees of lesion severity.
Conclusions: In vitro, CTA is accurate for the evaluation of bifurcation lesions. CTA was able to distinguish contrast-filled lumen even in severe obstructive lesions. These findings require further validation in the clinical setting.
Abbreviations
CAD: coronary artery disease
CT: computed tomography
CTA: computed tomography angiography
LOA: limits of agreement
MLA: minimal lumen area
MLD: minimal lumen diameter
%DS: percent diameter stenosis
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
RVD: reference vessel diameter
Introduction
Coronary computed tomography angiography (CTA) has been recommended as the first-line diagnostic test in patients with suspected coronary artery disease (CAD)1. Also, coronary CTA has been increasingly used as a tool for procedural planning prior to percutaneous coronary intervention (PCI)2. Furthermore, the use of coronary CTA during the work-up of patients with chest pain of recent onset has been shown to increase the prescription of optimal medical therapy (i.e., HMG-CoA reductase inhibitors and aspirin) and, when combined with blood flow simulation and virtual physiologic metrics such as fractional flow reserve (FFRCT), it has been shown to reduce unnecessary invasive coronary angiography3-5.
In complex CAD, coronary CTA might have advantages over invasive angiography. The three-dimensional (3D) reconstruction of the coronary tree allows complete rotation and angulation. In bifurcation lesions, this feature facilitates the visualisation of all bifurcation segments without foreshortening and overlapping, which are frequently encountered with invasive angiography6. In chronic total occlusions, coronary CTA permits evaluating the distal bed beyond the occlusion point, the length of the occlusion and extent, distribution and composition of the atherosclerotic plaque. These characteristics have been shown to predict procedural success and guide the PCI strategy7.
The comprehensive evaluation provided by coronary CTA might be of great value in patients with complex CAD. Nevertheless, the evidence of the accuracy of coronary CTA in these scenarios is scarce, since coronary CTA studies have focused on patients with non-complex lesions. This study aimed to validate quantitative coronary CTA analysis in complex angiographic lesions such as bifurcations and subtotal occlusions with a highly precise phantom as a reference.
Methods
STUDY DESIGN
Five Plexiglas phantoms with three bifurcation lesions each were designed and manufactured with a tolerance of <10 μm. For the design of the bifurcation phantoms, the diameters of the three bifurcation branches, the bifurcation angles, stenosis severity and location of stenosis were derived from the available relevant literature. All bifurcations met the natural fractal geometry of the coronary tree using Finet’s law to define the diameters of the distal main vessel (DMV) and the side branch (SB). Every bifurcation (n=15) had a lesion located within 3 to 6 mm from the point of bifurcation8. The series of bifurcation phantoms comprised 27 narrowed and 18 stenosis-free bifurcation segments with a minimal lumen diameter (MLD) ranging from 0.53 to 1.96 mm and RVD ranging from 1.40 to 4.00 mm. In addition, luminal stenoses were scaled up with increases of 10% from 40% to 80%, which corresponded with luminal areas ranging from 3.0 mm2 to 0.22 mm2. Taking into account the aforementioned literature-based specifications, the phantoms were designed (Pro/ENGINEER Wildfire v4; PTC, Needham, MA, USA). In this way, a digital 3D model of the luminal surface of the three bifurcations for each phantom was created. All bifurcations of the phantom were in the same plane. The geometric descriptions of the models were used to instruct a computer numerically controlled (CNC) milling machine (Fehlmann Picomax 60 HSC; Fehlmann AG, Seon, Switzerland) to mill these models into two Perspex (Plexiglas) blocks of 110×40×10 mm using a computer-aided design programme8.
CT ANGIOGRAPHY ACQUISITION AND ANALYSIS
The CT angiograms were acquired with a third-generation dual-source computed tomography scanner (SOMATOM Force; Siemens Healthcare, Forchheim, Germany). All phantoms were filled with a 3.75% solution of Iodixanol 320 (Visipaque™; GE Healthcare, Cork, Ireland) and sodium chloride at a concentration of 11.6 mg/ml. Acquisition parameters were set at a voltage of 70 kV and current of 500 mA. Reconstructed slice thickness was 0.6 mm with a 0.4 mm increment and using a Bv44 kernel and iterative reconstruction (ADMIRE 3). Data from coronary CTA were analysed off-line by an independent core lab (Cardialysis BV, Rotterdam, the Netherlands) using a validated cardiovascular analysis package for luminal analysis (QAngio CT Research Edition v3.0.14; Medis medical imaging systems bv, Leiden, the Netherlands); for angle assessment another software package was used (CardIQ Xpress 2.0, AW workstation; GE Healthcare). Manual extraction of the centreline of each vessel (i.e., main vessel and side branch) was performed followed by automatic segmentation of the vessel lumen; manual contour correction was not allowed. Single-vessel bifurcation analysis was performed with the manual division of the bifurcation segments (i.e., proximal and distal main vessel, and side branch)9,10. The minimal lumen area (MLA) and mean MLD were determined for each segment and compared to the phantom dimensions. The reference vessel diameter (RVD) was calculated as the average of the proximal and distal lumen diameters in the main vessel, and as the distal reference diameter for the side branch. The luminal percentage diameter stenosis (%DS) was calculated as (1–minimal lumen diameter/reference vessel diameter)*100. The proximal angle was defined as the angle between the proximal main vessel and side branch, whereas the distal angle was defined as the angle between the side branch and distal main vessel. In addition, each lesion at the MLD cross-section was qualitatively and quantitatively assessed for the visualisation of the contrast-filled lumen. To quantify the intensity of luminal signal, a point estimate of the region of interest (ROI) was used to measure the Hounsfield units (HU). Lesions were categorised as subtotal or total occlusion based on the visualisation of the lumen and the HU intensity.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation. Categorical variables are presented as counts and/or percentages. The signed differences were averaged; the mean of these signed differences (bias) was a measure of accuracy. The agreement for the measurements was performed using Bland-Altman analysis. As the phantom values were known, we chose to plot these instead of the average values on the x-axis against the differences on the y-axis11. Spearman’s correlation coefficient (rho) was used to assess the relationship between MLA and HU. All statistical tests were two-sided and a p-value <0.05 was considered statistically significant. All analyses were performed using SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA) and MedCalc Software version 14.12 (MedCalc, Ostend, Belgium).
The primary objective was to determine the diagnostic accuracy of quantitative CTA in assessing bifurcation lesion dimensions with the phantoms as a reference. The secondary objective was to determine the threshold of stenosis severity at which CTA is unable to detect contrast-filled lumen.
Results
BIFURCATION ANALYSIS
Five phantoms with 15 bifurcation lesions (45 segments) were analysed and included in this study (Figure 1). The Medina classification of the bifurcation lesions was 1,1,1 in four lesions; 1,1,0 in four lesions; 0,1,0 in three lesions; 1,1,1 in two lesions and 0,1,1 in two lesions. Overall, the accuracy of CTA for the assessment of MLD was –0.07 mm (limits of agreement [LOA] –0.75 to 0.61), and of RVD was 0.19 mm (LOA –0.25 to 0.63), which led to an accuracy for the %DS assessment of 8.2% (LOA –13.2 to 29.5) (Figure 2). A significant correlation was observed between the MLD and the mean difference in the assessment of the MLD (r=0.82, 95% CI: 0.70 to 0.90, p<0.0001). In lesions with MLD >1 mm, CTA overestimated the lesion severity (MLD bias 0.19 mm, LOA –0.09 to 0.47). Conversely, in lesions with MLD ≤1 mm, quantitative CTA analysis underestimated the lesion severity (MLD bias –0.48 mm, LOA –0.55 to –0.41). Moreover, in lesions with %DS ≥50%, quantitative CTA analysis underestimated the degree of stenosis (%DS bias 16%, LOA –5 to 37).
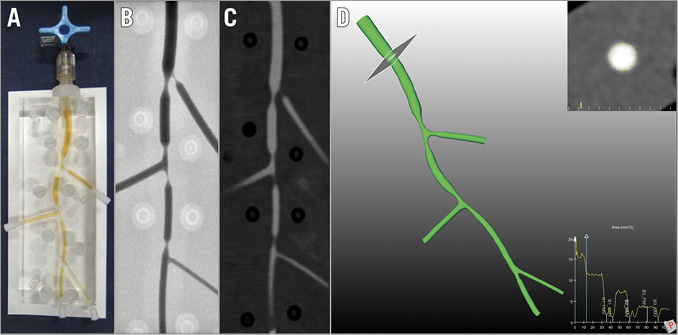
Figure 1. Bifurcation phantom. A) Plexiglas phantom with three bifurcations. B) Conventional angiography of a phantom. C) CTA of the phantom model. D) CTA-derived three-dimensional reconstruction of the phantom model. CTA: computed tomography angiography
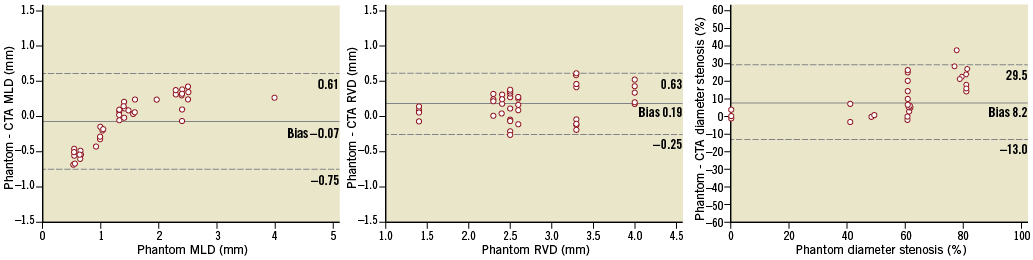
Figure 2. Mean differences in MLD (A), RVD (B), diameter stenosis (C). MLD: minimum lumen diameter; RVD: reference vessel diameter
A similar underestimation correlated to the lesion severity (i.e., MLD <1 mm and >1 mm) was observed. This finding was observed regardless of the location within the bifurcation (i.e., proximal vs. distal branches). This was associated with a trend towards a larger bias in the assessment of %DS at the distal main vessel (Figure 3). The accuracy for assessment of the proximal angulation of the bifurcation was 3.8° (LOA –7.7 to 15.3), whereas for the distal angle it was 8.1° (LOA –4.5 to 20.7).
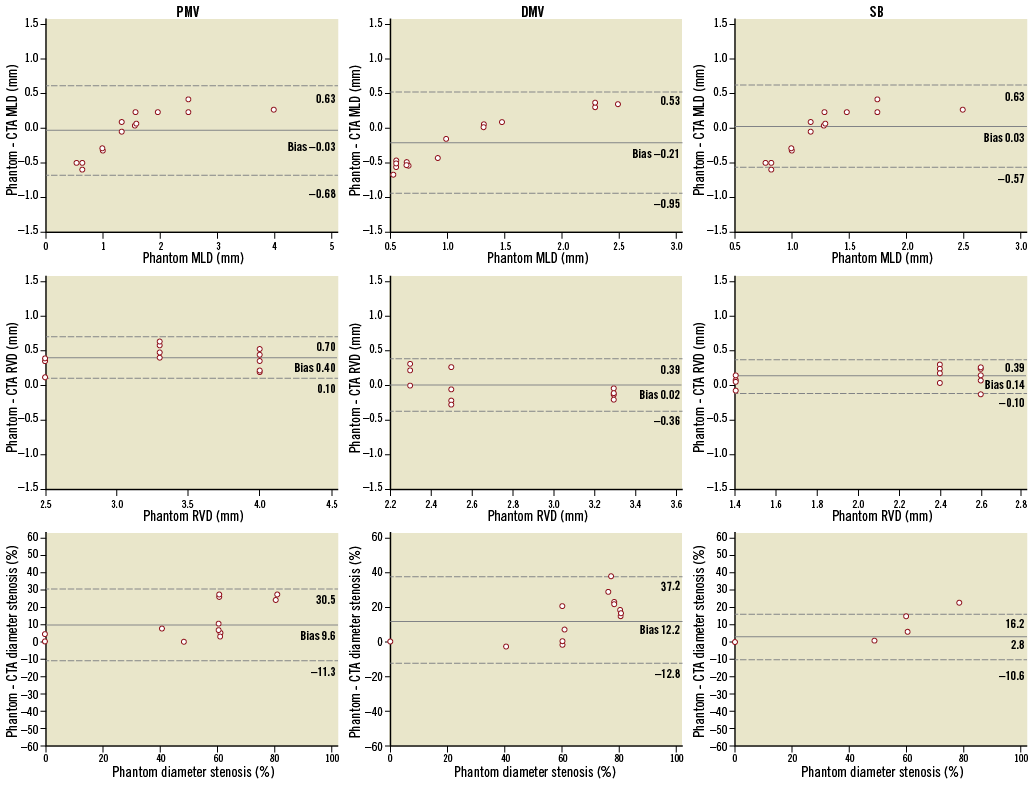
Figure 3. Mean differences in MLD, RVD and diameter stenosis of each bifurcation segment (i.e., proximal main vessel, distal main vessel and side branch). MLD: minimum lumen diameter; RVD: reference vessel diameter
GRADUAL STENOSIS ANALYSIS
Six lesions with a stepwise escalating stenosis with MLA of 3.0 mm2, 1.96 mm2, 1.37 mm2, 0.77 mm2, 0.32 mm2 and 0.22 mm2 were evaluated. Qualitative analysis showed a contrast-filled lumen in all degrees of lesion severity. The HU measured at the centre of the lumen showed a significant correlation with the severity of the stenosis (Spearman’s correlation coefficient=0.99, 95% CI: 0.87 to 0.99, p<0.001) (Figure 4). Regardless of the degree of stenosis, the HU measured at the centre of the lumen were ≥220 units. In addition, a significant correlation was found between the HU and the bias in the MLA evaluation.
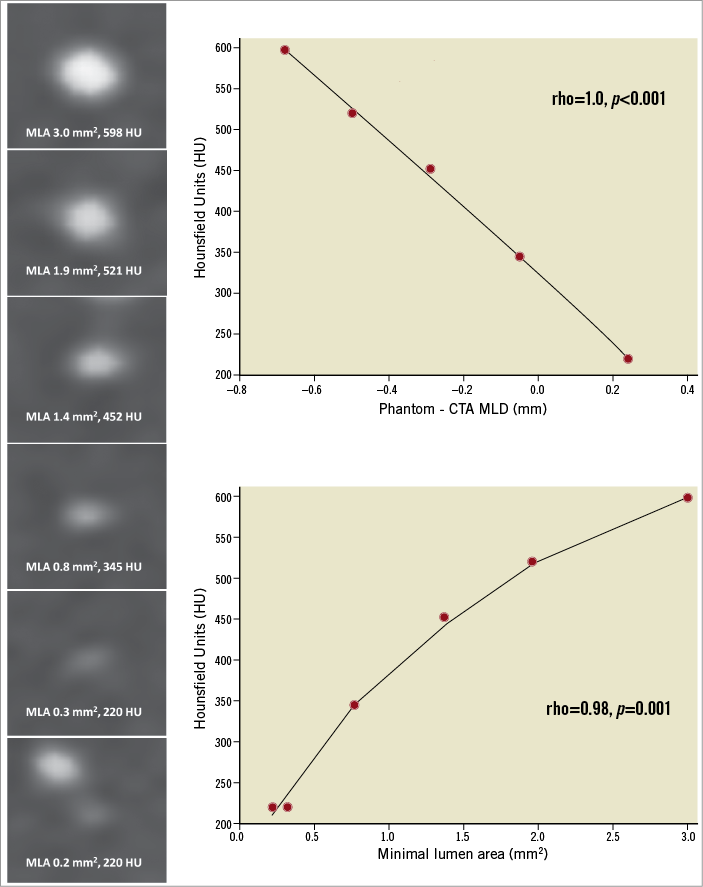
Figure 4. Gradual stenosis analysis. In the left panel, the cross-section of the gradual increase in stenosis severity with the corresponding MLA and HU. Right panel (top), the correlation between HU and bias in the assessment of MLD. Right panel (bottom), the correlation between HU and MLA. HU: Hounsfield units; MLA: minimum lumen area
Discussion
The main findings of this in vitro study can be summarised as follows: 1) quantitative CTA analysis is accurate for the assessment of MLD and RVD in bifurcation lesions, and overall bias is minimal; 2) severe stenoses are underestimated by quantitative CTA regardless of the lesion location within the bifurcation; and 3) CTA is able to identify luminal contrast enhancement even in severely obstructive lesions (up to 80% diameter stenosis).
Contemporary studies using multi-detector CT scanners have shown that quantitative coronary CTA has a high diagnostic accuracy for the assessment of coronary lesions. Miller et al demonstrated in non-complex coronary lesions that quantitative coronary CTA was accurate (AUC 0.93 [95% CI: 0.90 to 0.96]) for detecting stenosis ≥50% with invasive angiography QCA as a reference. Nonetheless, in lesions with a high degree of stenosis (i.e., %DS >70%), the accuracy of CTA was lower as compared to moderate lesions12. One of the major challenges of non-invasive QCA is the absence of dedicated bifurcation analysis which acknowledges the “step-down” anatomy observed in coronary bifurcations. Instead, a single-vessel quantitative analysis was used; this has been shown to be inaccurate for the quantitative assessment of bifurcation lesions10. In the present study, quantitative coronary CTA analysis was accurate to determine a clinically relevant parameter such as MLD (bias –0.07 mm, LAO –0.75 to 0.61) in all bifurcation segments. However, a higher bias in MLD measurement in coronary CTA was observed in the distal main vessel. This might be due to the localisation of the lesion in the distal main vessel immediately distal to the bifurcation where the contour tracing is affected by the take-off of the side branch. In addition, %DS was underestimated in high-degree stenoses irrespective of the location in the bifurcation. The calculation of %DS depends on the precise measurement of both MLD and RVD with the use of the interpolated reference line and dedicated bifurcation analysis, which is not available in the current CTA analysis software packages.
Reliable non-invasive assessment of lesions would constitute an advantage in the care of patients with known CAD. Previous studies have shown that coronary CTA overestimates the severity of moderate lesions13. In this study, lesions with MLD >1 mm which corresponded to a %DS between 40% and 60% were overestimated. Interestingly, in more severe lesions, with MLD ≤1 mm which corresponded to %DS >70%, quantitative coronary CTA systematically underestimated the severity of the stenosis. This difference might be partially explained by the limited spatial resolution of CTA to assess small luminal areas. The underestimation observed in the seven lesions with an MLD of 0.53 mm was systematic with a threshold of 1 mm for the MLD measurement with CTA.
Bifurcation angles are key factors in the planning of percutaneous interventions in bifurcation lesions. In complex bifurcations with a planned two-stent strategy, the bifurcation angle is a major determinant of the PCI technique14. Moreover, the occurrence of haemodynamic compromise of the side branch after main vessel stenting has been shown to be dependent on the distal bifurcation angle15. Dedicated bifurcation QCA packages have been shown to measure bifurcation angles accurately; nevertheless, the validity of 2D QCA is dependent on the angiographic view analysed and can be affected by foreshortening, plane magnification, and overlapping coronary structures16,17. The 3D nature of the CTA reconstruction represents a theoretical advantage for bifurcation angle assessment. A previous study using welded metal rod phantoms with six bifurcations at defined angles between 25° and 90° suggested a low bias (0.7±0.5°) in the assessment of the bifurcation angles18. In this study, CTA was accurate for the assessment of the bifurcation angles. An underestimation of 3.8° for the proximal angle and 8.1° for the distal bifurcation angle was found. This is similar to the bias reported using invasive coronary angiography. This finding supports the use of CTA for bifurcation angle assessment.
In the field of the percutaneous treatment of coronary chronic total occlusions, CTA plays a major role2. CTA guidance with high-resolution scanners has been shown to improve the success rate of chronic total occlusion PCI19. Furthermore, lesion characteristics such as stump morphology, length, the extent and distribution of calcium, tortuosity and negative remodelling have been found to be correlated with procedural success19,20. However, in some cases, the discrimination between a subtotal and total occlusion might be challenging. Recently, Choi et al described that a combination of characteristics, namely long occlusion length (cut-off ≥15 mm), higher distal transluminal attenuation gradient, side branches, blunted stump, cross-sectional calcification ≥50%, and collateral vessels, aided in distinguishing chronic total occlusion from subtotal occlusion21. One of the most reliable pieces of information provided by CTA to identify a patent vessel with a severe coronary obstruction is the presence of luminal enhancement. In this study, we were unable to determine a threshold below which no luminal contrast could be identified. Despite using a luminal area (i.e., 0.22 mm2) below the voxel resolution of the CT scanner, the contrast lumen could be qualitatively and quantitatively evaluated. Furthermore, this degree of lesion severity might not exist in clinical practice, given that the theoretical pressure gradient derived from the Gould equation through such stenosis would exceed 400 mmHg22. This result suggests that CTA is able to detect luminal contrast even in severe lesions. Of note, a negative correlation between the HU measured at the MLA and the bias in the quantitative assessment was found with an underestimation of the severity of the stenosis at the MLA site with higher HU. This finding, however, requires further investigation. Future quantitative CTA software algorithms might be improved by using different HU thresholds for luminal contour detection for different degrees of stenosis severity.
A decade ago, coronary CTA emerged as an alternative to non-invasive testing to investigate patients with suspected CAD12. It was shown to be a useful tool to exclude obstructive coronary lesions. Recently, coronary CTA has been shown to have a similar diagnostic accuracy to invasive angiography to detect obstructive non-complex lesions23. The present study further extends this knowledge, providing in vitro evidence of the accuracy of CTA in complex lesion subsets such as bifurcation lesion and subtotal occlusions. The results of this study might lay the foundations for extending the use of coronary CTA to complex CAD.
Limitations
The main limitation of this in vitro study is the fact that the image acquisition was performed without simulating cardiac motion. Also, a chest phantom which could mimic the tissue attenuation of the thoracic wall was not used. This experimental set-up might have improved the image quality and CTA accuracy. Second, image acquisition was performed with a predefined contrast medium concentration with a low kV and high mAs which might differ from clinical practice. Furthermore, for image reconstruction we used a specific convolution kernel and iterative reconstruction level. Changing these parameters will potentially result in a different outcome. Third, although the precisely manufactured phantom used in this study resembles the anatomy observed in human bifurcation lesions, plaque components (e.g., calcium) which have been shown to impact on the luminal accuracy assessment were not included. Fourth, the image acquisition was performed with one type of CT scanner and the quantitative images analysed by one software package; therefore, the generalisation of these findings to other scanners and QCA algorithms requires further study. Nevertheless, the achievement of the final results reinforces the accuracy of CTA in the evaluation of complex lesions, at least in ideal image quality scenarios, though replication of such findings in vivo is needed.
Conclusions
Computed tomography angiography is accurate for the in vitro quantitative evaluation of bifurcation lesions. Moreover, CTA was able to distinguish contrast-filled lumen even in severely obstructive lesions. These findings require further validation in the clinical setting.
| Impact on daily practice Coronary computed tomography angiography (CTA) investigation is recommended in low-risk patients to exclude obstructive coronary artery disease; however, its utilisation in patients with established CAD is currently under investigation. The present study provides in vitro evidence of the accuracy of coronary CTA for the quantitative angiographic assessment of bifurcation lesion and subtotal occlusions. |
Guest Editor
This paper was guest edited by Daniele Andreini, MD, PhD; Centro Cardiologico Monzino, IRCCS, Milan and Department of Clinical Sciences and Community Health, Cardiovascular Section, University of Milan, Milan, Italy.
Funding
Pie Medical Imaging funded the design and construction of the bifurcation phantoms.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

