
Abstract
Aims: Randomised trials comparing transcatheter aortic valve replacement (TAVR) with surgical aortic valve replacement (SAVR) have included mainly elderly patients >80 years. The authors aimed to investigate comparative in-hospital outcomes of younger patients <75 years old undergoing transfemoral (TF) TAVR or isolated SAVR.
Methods and results: A total of 6,972 patients aged 65-74 years undergoing TF-TAVR or SAVR between 2013 and 2014 were identified from the observational German Quality Assurance Registry on Aortic Valve Replacement (AQUA), which comprises all TAVR and SAVR procedures performed in Germany. Analyses were performed for the overall unmatched cohort as well as for 1,388 propensity-matched patients. Overall, 82.4% of patients <75 years needing treatment for aortic valve stenosis received SAVR. Patients undergoing TF-TAVR were older and had more comorbidities with higher predicted risk of death. After propensity matching, in-hospital mortality (1.3% vs. 1.9%, p=0.39), stroke/TIA (1.0% vs. 2.1%, p=0.09), and myocardial infarction (0 vs. 0.3%, p=0.16) were not different after TF-TAVR or SAVR. Postoperative delirium was more frequent after SAVR (8.9% vs. 2.4%, p<0.001), whereas the need for a new pacemaker was four times higher after TF-TAVR (13.3% vs. 3.5%, p<0.001).
Conclusions: Younger patients <75 years old undergoing TF-TAVR or SAVR had similar outcomes with the exception of a more frequent need for new pacemaker implantation and a less frequent incidence of postoperative dialysis and delirium in TF-TAVR patients. Whether these similar in-hospital outcomes are replicable in the longer-term events in TF-TAVR and SAVR remains to be proven in future studies.
Abbreviations
AS: aortic stenosis
CC: creatinine clearance
CCS: Canadian Cardiovascular Society
GAV: German Aortic Valve
log.ES: logistic EuroSCORE
LV: left ventricular
NOTION: Nordic Aortic Valve Intervention trial
NYHA: New York Heart Association
PARTNER: Placement of Aortic Transcatheter Valves trial
RCT: randomised controlled trial
SAVR: surgical aortic valve replacement
TAVR: transcatheter aortic valve replacement
TF: transfemoral
TIA: transient ischaemic attack
Introduction
Supported by strong evidence from high-quality randomised controlled trials (RCT), transcatheter aortic valve replacement (TAVR) has become the standard treatment for most inoperable or high surgical risk patients with severe symptomatic aortic valve stenosis1-3. More recent RCT have suggested TAVR to be – at least – non-inferior to surgical aortic valve replacement (SAVR), even in those deemed at intermediate risk for open heart surgery4,5. So far, TAVR trials have included mainly elderly patients: mean age was highest in the inoperable cohort of the Placement of Aortic Transcatheter Valves (PARTNER) I RCT at 83.1±8.6 years1, but was still 81.6 years4 and 79.8 years5 in the more recent intermediate-risk trials. Even in the randomised all-comer (low-risk) Nordic Aortic Valve Intervention (NOTION) trial, the mean age was still 79.1 years6. It has therefore been questioned whether the favourable or comparable TAVR results versus those observed with SAVR reported in these trials in older patients are applicable to younger patients with symptomatic aortic valve stenosis (AS). Yet, despite this lack of evidence, recent registry data have indicated that there is a trend for an increasing number of lower-risk and younger patients with symptomatic aortic stenosis being subjected to TAVR over SAVR7.
We analysed observational data from the mandatory German Quality Assurance Registry on Aortic Valve Replacement (“AQUA” registry) to evaluate comparative outcomes of all younger patients <75 years old undergoing isolated SAVR versus transfemoral TAVR in Germany in 2013 and 2014.
Methods
For the present analysis, we studied the complete 2013 and 2014 data sets of the German Quality Assurance Registry on Aortic Valve Replacement of the Federal Joint Committee, which was led until 31 December 2014 by the independent Institute for Applied Quality Improvement and Research in Health Care (AQUA, Göttingen, Germany). Since 2015, the lead of the German Quality Assurance Registry has changed to the newly founded Federal Institute for Quality and Transparency in Health Care (IQTIG, Berlin, Germany). Results for 2015 and 2016 have been published online, but data are not yet available for further analysis. Details of the nationwide “AQUA” registry have been described previously8. In brief, the registry comprised all in-patient SAVR and TAVR procedures performed in Germany, as participation was mandatory and linked to reimbursement. Events were predefined in an elaborate form completion guide and self-reported by the sites using these definitions. Data were pooled in a nationwide database and controlled for quality by a validated system.
For the present analysis, all patients aged ≥65 and <75 years undergoing isolated SAVR or TAVR were selected. Patient-level data analysis was limited to transfemoral (TF) TAVR patients only to avoid selection bias from including transaortic or transapical TAVR patients who differed significantly from transfemoral TAVR patients with respect to risk profiles and comorbidities.
In addition to the logistic EuroSCORE I (log.ES I), the log.ES II and the German Aortic Valve (GAV) score were used as risk prediction tools for estimation of in-hospital mortality. The GAV score was calculated retrospectively based on data from patients undergoing isolated SAVR or TAVR in 2008 and updated in 20139. Patients were categorised according to the log.ES I as low (log.ES I <10%), intermediate (log.ES I 10%-20%) and high surgical risk (log.ES I >20%) patients10.
STATISTICAL ANALYSIS
Statistical analysis was performed using IBM SPSS for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean±standard deviation and compared using the Student’s t-test including Levene’s test. Categorical variables are presented as frequencies in percent and compared using either Fisher’s exact test (for cell count <5) or the chi-square test as appropriate. A p-value <0.05 was considered statistically significant. Propensity scores were calculated by way of a logistic regression model from pertinent baseline patient characteristics (covariates). Those covariates comprised all items of the EuroSCORE II: age, gender, New York Heart Association (NYHA) class (II, III, IV), Canadian Cardiovascular Society (CCS) class IV, insulin-dependent diabetes mellitus, extracardiac arteriopathy, chronic pulmonary dysfunction, neurological or musculoskeletal dysfunction severely affecting mobility, dialysis, creatinine clearance (CC ≤50 ml/min, CC 50-85), critical preoperative state, left ventricular (LV) function (≤20%, 21-30%, 31-50%), pulmonary hypertension, recent myocardial infarction, urgency (elective, urgent). Treatment modality was the dependent variable in the logistic regression equation, i.e., the propensity score indicates the likelihood of the patient belonging to the TF-TAVR cohort as opposed to the SAVR group. Of note, the log.ES I, log.ES II and GAV score were not among the covariates used to calculate the propensity score. Patients from the TF-TAVR and SAVR cohorts were subsequently randomly matched for propensity scores differing not more than ±0.00005. This approach matched sets of 694 SAVR cases and 694 TF-TAVR cases, with 489 pairs having exactly the same propensity score. According to Austin11, interferences about treatment effects are valid only if in the matched sample, i.e., subjects in the treatment groups have a similar distribution of measured baseline covariates. For the above-listed covariates for the two treatment samples, the standardised differences were all <0.1, suggesting sufficient homogeneity of the two treatment samples.
Additionally, we analysed the 489 pairs with exactly the same propensity scores in the setting of a sensitivity analysis. The results corresponded to the results of the overall 694 pairs of propensity score matching, so we decided to present here only the results for the propensity score matching.
Results
UNMATCHED OVERALL COHORT
Between January 2013 and December 2014, a total of 6,972 patients aged 65-74 years underwent isolated SAVR or TF-TAVR in Germany (Table 1). The majority of these patients (5,708/6,972, 82.4%) underwent SAVR. Patients undergoing TF-TAVR were older and had more comorbidities, resulting in higher predicted risk of operative mortality than SAVR patients (Table 1). Table 2 shows the Heart Teams’ reasons for selecting TF-TAVR over SAVR in the individual patients. More than 95% of patients undergoing SAVR were at low surgical risk with a log.ES I <10% (Figure 1A). Slightly more than half of the TF-TAVR patients (55.5%) were at low risk, while 28.1% were considered intermediate-risk, and the remaining 16.4% high-risk with a log.ES I >20%.
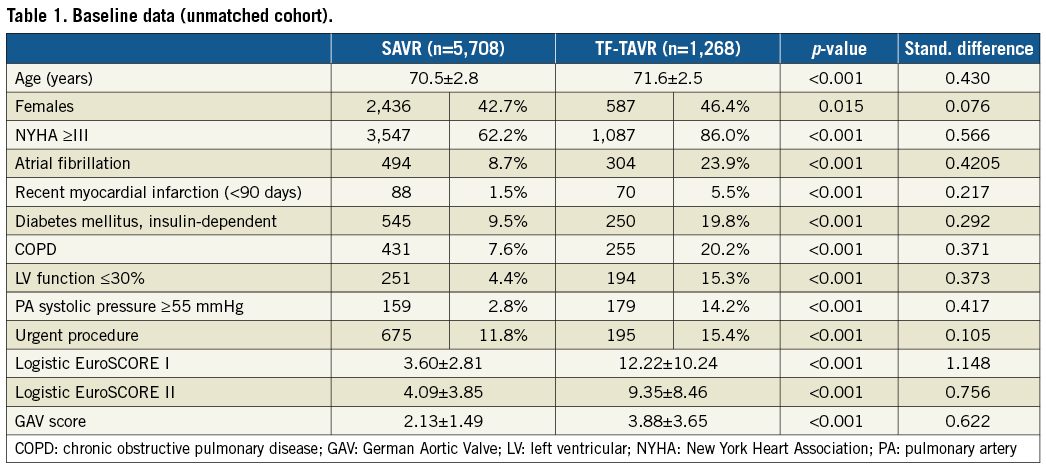
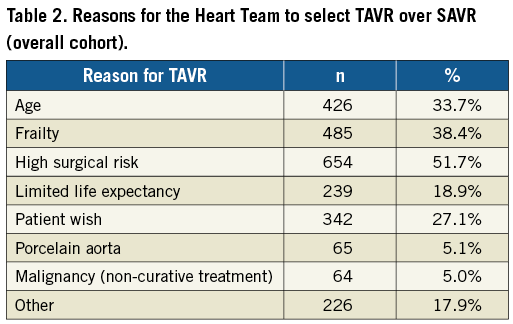
Figure 1. Distribution of predicted surgical risk classes according to logistic EuroSCORE I among TF-TAVR and SAVR patients. A) Unmatched overall cohort. B) Propensity-matched cohort.
Rates of in-hospital mortality were higher among those undergoing TF-TAVR (3.2% vs. 1.3%, p<0.001), while neurologic events were comparable between TF-TAVR and SAVR patients. TF-TAVR patients were less likely to have postoperative delirium requiring treatment, but more often received new permanent pacemaker implantation after the procedure than SAVR patients (Table 3). After SAVR, the number of patients requiring dialysis more than doubled as compared to baseline, while the number of patients on dialysis undergoing TF-TAVR was unchanged preoperatively versus postoperatively. Length of postoperative stay in hospital was shorter after TF-TAVR (10.1±8.1 days vs. 11.4±7.4 days, p<0.001). After TF-TAVR, more patients were discharged directly home rather than to another hospital or rehabilitation unit (65.5% vs. 37.7% after SAVR) (Table 3).
PROPENSITY-MATCHED COHORT
The propensity-matched cohort (according to log.ES II) included 694 SAVR and 694 TF-TAVR patients with a mean age of 71.7 and 71.5 years, respectively (Table 4). Patients were well matched with respect to comorbidities. Nevertheless, the predicted surgical risk by log.ES I was still higher among TF-TAVR patients (Figure 1B), while log.ES II and GAV score were not different between the groups. The vast majority of patients were at low risk, with a log.ES I <10% in 95.5% of SAVR patients and 86.3% of TF-TAVR patients. Only 0.4% and 1.9%, respectively, were at high surgical risk.
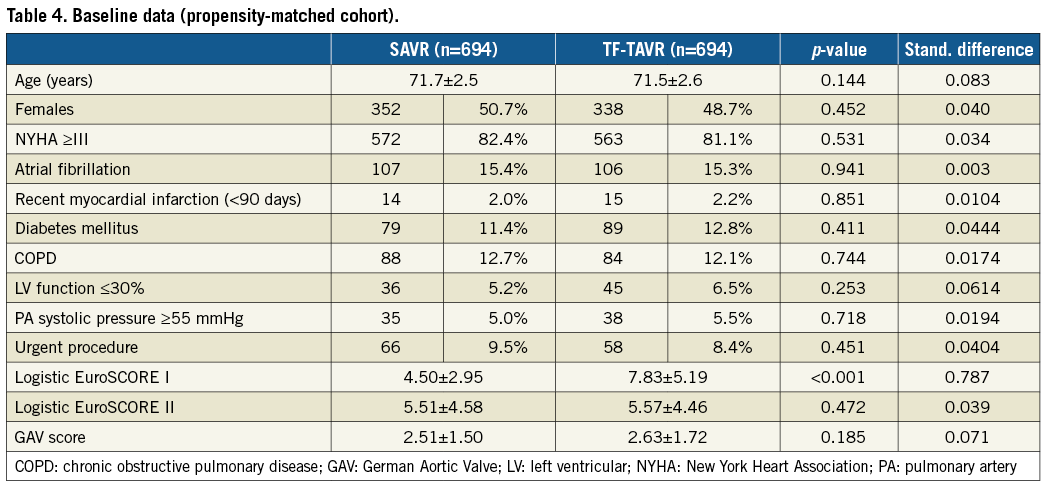
In-hospital mortality as well as rates of stroke/TIA and myocardial infarction were not different between the two groups (Table 5, Figure 2). SAVR patients had a more than threefold higher rate of postoperative delirium than TF-TAVR patients (8.9% vs. 2.4%, p<0.001). Similarly, the increase in patients needing dialysis was more pronounced (2.5% pre-op versus 4.8% post-op) for SAVR compared with TF-TAVR (1.6% pre-op versus 2.3% post-op). In contrast, TF-TAVR patients had a more than fourfold increase in the need for a new pacemaker implantation after the procedure compared with SAVR patients (13.3% vs. 3.5%, p<0.001). Length of postoperative hospital stay was shorter after TF-TAVR (9.5±7.9 days vs. 12.5±10.7 days after SAVR, p<0.001). More patients were discharged home rather than to another hospital or rehabilitation unit after TF-TAVR (70.5% vs. 33.1%) (Table 5).
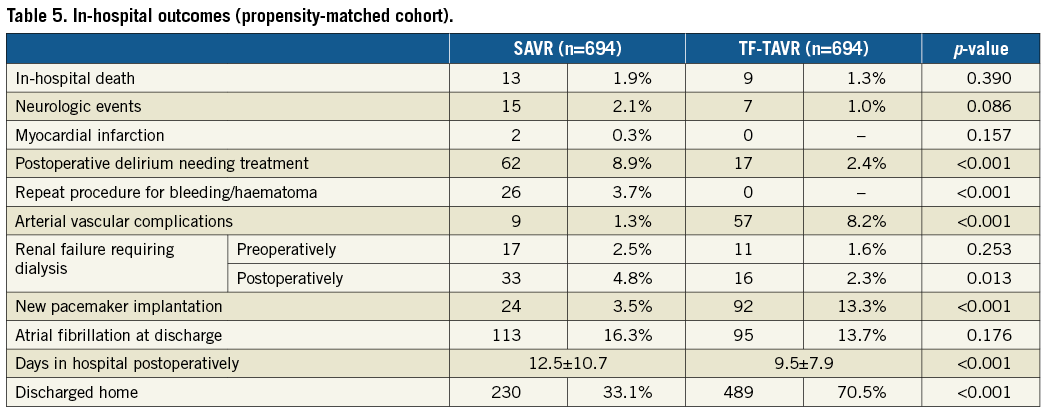
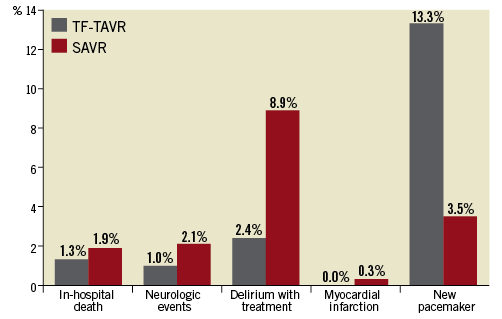
Figure 2. Outcomes after TF-TAVR and SAVR for the propensity-matched cohort.
Discussion
This registry with its real-world data is the first to evaluate comparative outcomes after isolated SAVR or transfemoral TAVR in younger patients aged 65-74 years. The findings suggest similar rates of in-hospital mortality and post-procedural stroke/TIA between the two treatment strategies. The rates of postoperative delirium requiring therapy and postoperative dialysis were significantly higher after SAVR, whereas TAVR patients had a fourfold higher risk of requiring new pacemaker implantation. Finally, TAVR patients were more often discharged home than SAVR patients who were more likely to be discharged to a rehabilitation unit or to other hospitals. These findings are largely consistent with the results of previous RCT that included mainly elderly patients >80 years2-6.
For patients aged >80 years, the randomised PARTNER trials (for balloon-expandable transcatheter heart valves [THV]) as well as the CoreValve U.S. High Risk trial and, most recently, the SURTAVI trial (for the self-expanding THV) have demonstrated TAVR to be equivalent (or better) as compared to SAVR, findings that were consistent for all risk categories1-6. Supported by this evidence, annual TAVR numbers are increasing worldwide. In Germany, numbers of TAVR have increased >20-fold since 20087. In 2016, 17,065 patients underwent TAVR as compared to 9,579 patients undergoing isolated SAVR12. Most patients >80 years of age needing treatment for AS were treated with TAVR (2016: 92%)12, suggesting rapid adoption of the favourable results of the randomised TAVR trials into clinical practice in Germany.
The 2017 Valvular Heart Disease Guidelines of the European Society of Cardiology13 favour TAVR over SAVR in non-low-risk patients >75 years old. For younger patients <75 years old, SAVR still remains the preferred therapy. So far, there are no randomised data for this age group available. Our analysis of all SAVR and TAVR procedures in Germany shows that indeed most (82.4%) of the patients <75 years old who needed treatment for AS received SAVR. These patients were predominantly at low predicted risk of periprocedural death with >95% having a log.ES I <10%. Younger patients <75 years old receiving TAVR had more comorbidities and were at higher risk (1/3 intermediate risk, 1/5 high risk). Yet, slightly more than half of the TAVR patients were considered low-risk, at least according to their log.ES. It is likely that other risk factors or comorbidities not captured in the log.ES may have led the Heart Team to decide in favour of TAVR, based on an individual risk assessment.
Propensity-matched analysis showed that younger patients <75 years old had excellent outcomes that were similar with both SAVR and TAVR. Specifically, in-hospital mortality rates were low, ranging between 1% and 2% and similar for both SAVR and TAVR. The low mortality in both cohorts was reflective of the fact that more than 95% of SAVR and 86% of TAVR patients had a log.ES I <10% (low surgical risk). Our results corroborate previous RCT and non-randomised registries. In the all-comer NOTION trial which included patients at low risk (mean log.ES I 8.4%) but older age (79 years), 30-day mortality was not different between SAVR and TAVR (3.7% vs. 2.1%)6. In the more recent PARTNER 2A trial that randomised elderly patients at intermediate risk, 30-day mortality rates were not different between SAVR and TAVR (4.1% vs. 3.9%, p=0.78)4. In the prospective, non-randomised PARTNER II SAPIEN 3 registry, 30-day mortality among elderly, intermediate-risk patients was 1.1% and thus very similar to the present analysis (1.3%)14.
Since the early PARTNER I trial, neurologic events have become a major concern for patients undergoing TAVR15, an outcome and its sequelae that may be even more dreadful in younger patients with longer life expectancies. Our analysis of observational data suggested that rates of post-procedural neurologic events are low and may not be different between SAVR and TAVR patients. Numerically, neurologic events were 50% lower in TAVR patients, although not statistically different compared with those undergoing SAVR. There was a higher rate of postoperative delirium requiring therapy after SAVR in the present study, an event that is not without adverse consequences16,17. Our analysis is thus largely consistent with the results of recent RCT that have not shown increased stroke risk with TAVR compared with SAVR4,5. However, overall rates of neurologic complications were lower (by 50%) than in previous RCT3,5, but similar to the recent non-randomised PARTNER II SAPIEN 3 registry14. This difference may be explained by the lower age and risk profile of the patients in the present analysis. Nevertheless, we cannot exclude under-ascertainment or underreporting of neurologic events in this observational registry as compared to RCT with rigorous postoperative neurology assessment which is perhaps likely to reveal a higher incidence of both clinically significant and insignificant strokes due to independent adjudication of adverse events18.
Not all adverse events were similar or lower in TAVR compared with SAVR. Dependent on the type of THV used, rates of new pacemaker implantation after TAVR varied between 10.1% and 32%19, but have been consistently higher than after SAVR in the randomised trials including mainly elderly patients >80 years. In the present analysis of younger patients <75 years old, rates of new pacemaker implantation were still fourfold higher after TAVR than after SAVR, but similar to a recent analysis of elderly US patients (median age of 82 years) undergoing this procedure (13.3% vs. 12.8%)18. For elderly patients, it has been shown that mortality up to 24 months was similar between those who received a new pacemaker after TAVR and those who did not5,20. Nevertheless, the higher rates of permanent pacemaker after TAVR remain of concern, particularly for younger patients with longer life expectancy and thus higher chances of experiencing technical complications of the pacemaker device or its electrodes or developing long-term consequences of right ventricular pacing. Previous analyses found reduced left ventricular ejection fraction and impaired LV unloading in patients with new pacemaker after TAVR which may be explained by ventricular dyssynchrony due to chronic right ventricular pacing21,22. Patients with a new pacemaker after TAVR had higher re-hospitalisation rates which may be linked to development of heart failure in these patients22,23.
After valve replacement, 2/3 of SAVR patients were discharged to other hospitals or rehabilitation units, while 70% of TAVR patients were discharged home. This, together with less postoperative delirium and shorter hospital stays, suggests that recovery after the less invasive TAVR procedure was less demanding. Rates of discharge home after TAVR were identical to a previous analysis of US real-world TAVR patients18. Although patients of the present analysis were on average 10 years younger than the US cohort (median age: 82 years), only 33% of SAVR patients were discharged home as compared to 41% of the US SAVR patients18. This may most likely reflect differences in the healthcare systems, as more patients from the present analysis were sent to other hospital or rehabilitation units than in the US cohort (62.7% vs. 41.2%).
Limitations
The database has some limitations. First, as is inherent to any observational registry, some data were missing or not collected. The association of this missing information with patient outcomes cannot be excluded. Importantly, the degree of residual paravalvular leakage after TAVR/SAVR is not assessed within the AQUA registry. Thus, we are unable to provide any insights into its potential clinical impact, particularly on longer-term outcomes. Second, we cannot exclude incompleteness of data which is inherent to this type of registry without routine on-site data verification. However, this would affect both SAVR and TAVR patients. Third, this data set contains only data on short-term outcomes limited to the in-hospital period. Unlike SAVR where the long-term valve durability and impact on long-term outcomes including mortality and quality of life have been well documented in younger patients, this information is lacking in younger patients undergoing TAVR. Future studies are needed to evaluate whether the benefit of a less invasive procedure such as TAVR with favourable short-term outcomes would be similarly evident in terms of long-term valve durability and long-term outcome domains such as mortality and quality of life.
Conclusions
This propensity-matched analysis of 1,388 patients <75 years old undergoing SAVR or TAVR suggests similar in-hospital outcomes but less demanding recovery after TAVR. Further randomised studies are needed in younger patients to confirm these favourable in-hospital results during the long-term course.
| Impact on daily practice In current practice, the majority of younger patients <75 years old needing treatment for aortic valve stenosis receive surgical aortic valve replacement (SAVR). Individual patients <75 years old are selected for transcatheter aortic valve replacement (TAVR) by the Heart Team according to comorbidities or other factors that increase the risk of open heart surgery. This propensity-matched analysis suggests similar rates of in-hospital mortality or stroke after SAVR or TAVR in younger patients, with faster recovery after TAVR but fourfold increased rates of new pacemaker implantation. |
Funding
This analysis was partly supported by an unrestricted grant from the German Cardiac Society.
Conflict of interest statement
K. Bestehorn has received a grant from the German Cardiac Society during the conduct of the analysis. The other authors have no conflicts of interest to declare.

