
Abstract
Aims: The aim of this study was to evaluate the impact of pulmonary ridge (PR) coverage on both clinical and imaging follow-up outcomes in patients undergoing left atrial appendage occlusion (LAAO).
Methods and results: The study included consecutive patients with non-valvular atrial fibrillation who underwent LAAO with disc and lobe devices. Patients were classified into two groups according to the PR coverage. A total of 147 patients were included. Among these, the PR was covered in 109 (74%) and uncovered in 38 (26%). Successful implantation was achieved in 98.6%. No differences in procedural outcomes were observed between the groups. The rate of procedural major adverse events was 3% (only major bleedings and/or vascular access complications). No device embolisation, cardiac tamponade or in-hospital mortality was observed. After a mean follow-up of 1.77±2.2 years, the annualised ischaemic stroke and major bleeding rate was 1.3%/year and 6.5%/year, respectively, without differences between groups. At follow-up, patients with a covered PR presented a lower incidence of device-related thrombosis (DRT) (1%) than those with an uncovered PR (27%); p<0.001. In multivariable analysis, the presence of PR coverage emerged as an independent predictor of DRT.
Conclusions: Pulmonary ridge coverage was associated with a lower incidence of DRT after LAAO. Procedural and follow-up clinical outcomes did not differ between covered PR and uncovered PR patients.
Introduction
Left atrial appendage occlusion (LAAO) seems to be a procedure which is increasing progressively worldwide1,2. Like other interventional structural techniques, LAAO is a relatively novel procedure that is associated with a significant learning curve in terms of patient selection, baseline imaging, procedural technique and follow-up outcomes3,4,5. In fact, several publications have shown a progressive reduction in the number of procedural complications such as pericardial effusion or device embolisation with increasing operator experience2,6,7,8. More recent reports have focused on other technical follow-up outcomes such as device-related thrombosis (DRT)9,10,11,12. Although DRT was not initially associated with stroke at follow-up, with the growth in the number of LAAO procedures, the presence of DRT has been linked to a higher incidence of stroke/transient ischaemic attack (TIA) after LAAO9,10. DRT seems to be a multifactorial process that can be prevented not only with antithrombotic treatment but also with some procedural considerations. Aminian et al11 have recently shown that >80% of DRTs with the Amulet device (AMPLATZER™ Amulet™; Abbott Vascular, Santa Clara, CA, USA) were located in the area below the pulmonary ridge (PR). Although not yet proven, the absence of PR coverage has been suggested as a potential risk factor for DRT11,13. The presence of low blood velocities and flow turbulence might create a prothrombotic environment in this area that may translate into a higher risk of DRT13,14. For this purpose, the present study sought to evaluate the impact of PR coverage in patients undergoing LAAO on both clinical and imaging follow-up outcomes.
Methods
The study included consecutive patients with non-valvular atrial fibrillation (NVAF) who underwent LAAO in a high-volume institution between 2012 and March 2020. In order to evaluate the role of PR coverage and avoid device biases, we included only those patients with disc and lobe LAAO device implantation. For the purposes of the study, patients were divided into two groups according to the PR coverage - covered PR and uncovered PR. Pulmonary ridge coverage was defined as the presence of the disc beyond the PR (Figure 1A). Two expert physicians considered all LAAO cases and classified patients according to the PR coverage. No disagreement was reported in any of the cases.
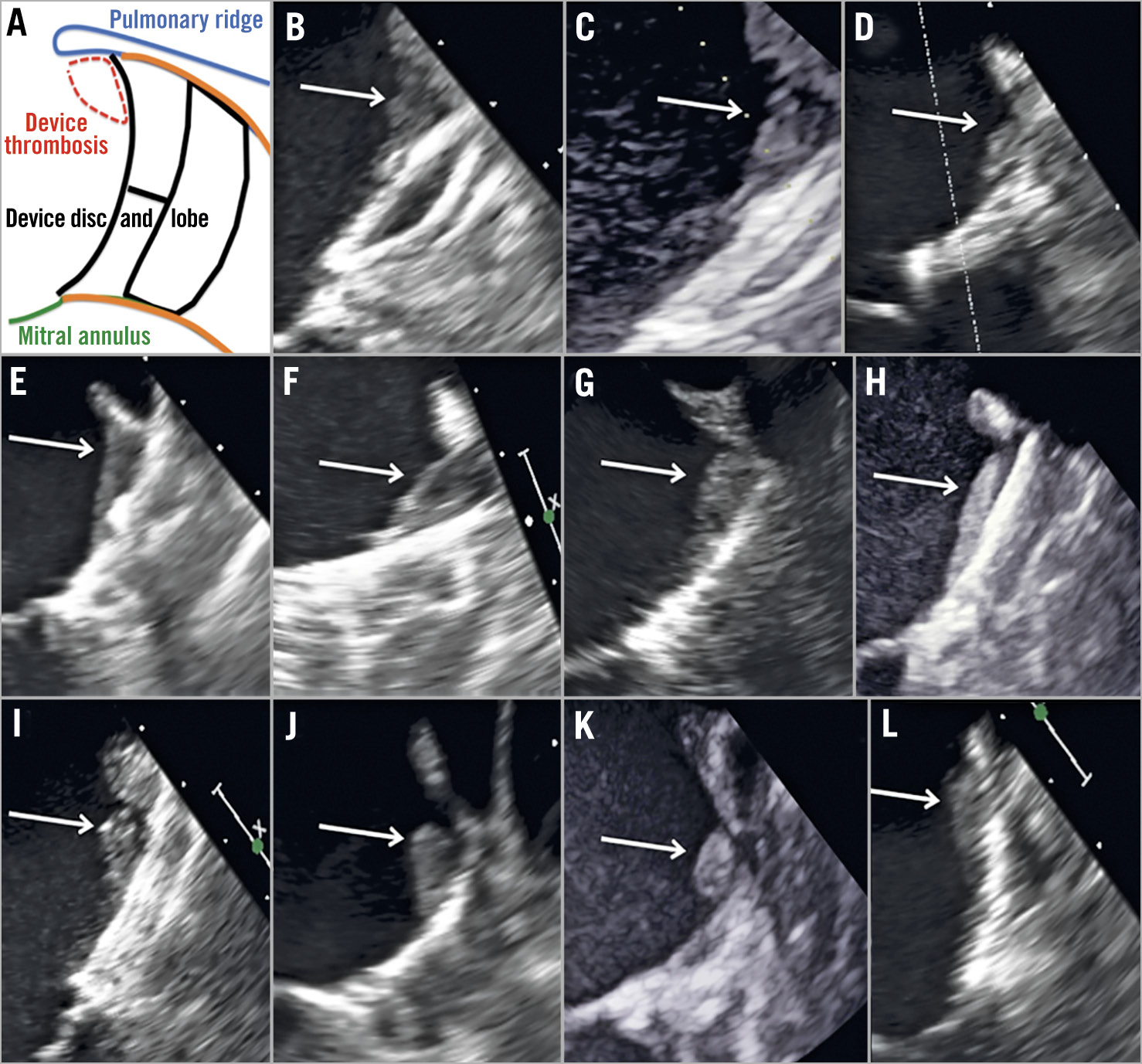
Figure 1. 2D-TEE images of every single patient with device-related thrombosis. A) Explanatory figure of thrombus location. B)-K) Device-related thrombus of patients with an uncovered PR. L) Device-related thrombus of a patient with a covered PR.
From the procedural point of view, PR coverage was always aimed for but sometimes not possible for different reasons: deep device implantations, PR protrusion, risk of device embolisation or just to reduce the number of device recaptures to minimise device/catheter manipulation inside the LAA.
Preprocedural imaging was performed mainly with two-dimensional transoesophageal echocardiography (2D-TEE) and three-dimensional (3D)-TEE. Only in patients with an intolerance to a TEE probe was computed tomography (CT) carried out. Details regarding the LAAO procedure and special features of the occlusion devices have been published elsewhere5,15. Prospectively collected data were transferred to a dedicated database. The study was approved by the institutional committee and all subjects gave consent. The study conformed to the guiding principles of the Declaration of Helsinki.
Procedural success was defined as successful implantation of the device in the LAA4. Procedural adverse events and major adverse events (MAEs) were reported according to the Munich consensus paper4. Major bleeding events were defined as type 3 or greater on the Bleeding Academic Research Consortium (BARC) scale16. Clinical follow-up was carried out by patient visit, medical report review and phone contact. Adverse events reported at follow-up included death (cardiovascular and non-cardiovascular), stroke, systemic embolism, and major bleeding. Since the estimated haemorrhagic risk varied among patients, antithrombotic treatment after LAAO was left to individualised risk estimation by the treating physician.
Device efficacy to prevent stroke, TIA, and systemic embolism was tested by comparing the actual event rate at follow-up with the predicted event rate using the CHA2DS2-VASc score17. Individual patient annual risk was recorded and the average annual risk for the whole study population was calculated. The total number of thromboembolic events (defined as stroke, TIA, and systemic embolism) during both periprocedural and follow-up periods was divided by the total patient years of follow-up and was multiplied by 100 in order to get the actual annual rate of thromboembolism. Thromboembolism reduction was calculated as follows: (estimated % – actual % event rate)/estimated % event rate.
IMAGING FOLLOW-UP
The first imaging follow-up test (generally 2D-TEE) was usually performed between the 6th and 12th week after the procedure. Following expert recommendations18, a second 2D-TEE was performed between the 6th and 12th month after LAAO. All imaging tests were prospectively analysed to explore the existence of DRT and peri-device leak (PDL). Definitions of DRT and PDL followed the Munich consensus paper4.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies (percentages), assessing the differences by chi-square test (or Fisher’s exact test when necessary). Continuous variables are presented as a mean±standard deviation (SD) or as a median (interquartile range). The Kolmogorov-Smirnov test was applied to ensure normal distribution. Continuous variables were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate. For the study of predictors of DRTs, we obtained the odds ratio (OR) with associated 95% confidence interval (CI) from univariable and multivariable logistic regression analysis. For all analyses, a two-tailed p-value <0.05 was used as the criterion for statistical significance. Follow-up was considered to end at the date of the last follow-up. Analyses were performed using Stata software, release 14.0 (StataCorp LP, College Station, TX, USA).
Results
Throughout the inclusion period, 152 patients underwent LAAO intervention. LAAO was not possible in two patients and the WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) (lobe-only device) was used in another three. Finally, 147 patients were included for the present analysis. Among them, the PR was covered in 109 (74%) and uncovered in 38 (26%).
Baseline characteristics are shown in Table 1. As shown, variables were balanced but there was a higher prevalence of patients with CHA2DS2-VASc ≥4 in the covered PR group.
Successful implantation was achieved in 98.6% of patients. As shown in Table 2, LAAO was conducted using the AMPLATZER™ Cardiac Plug (10%) (Abbott Vascular), AMPLATZER Amulet (75%) and LAmbre™ (15%) (Lifetech Scientific [Shenzhen] Co., Ltd., Shenzhen, China). No differences in procedural outcomes were observed among the groups. The rate of procedural MAEs was 3% (only major bleedings and/or vascular access complications); no device embolisation, cardiac tamponade or mortality was reported. As shown in Figure 2, the rate of PR coverage improved from 60% to 87% as the experience of operators increased. Dual antiplatelet therapy (44%) was the most used post-procedural antithrombotic therapy without significant differences among the groups.
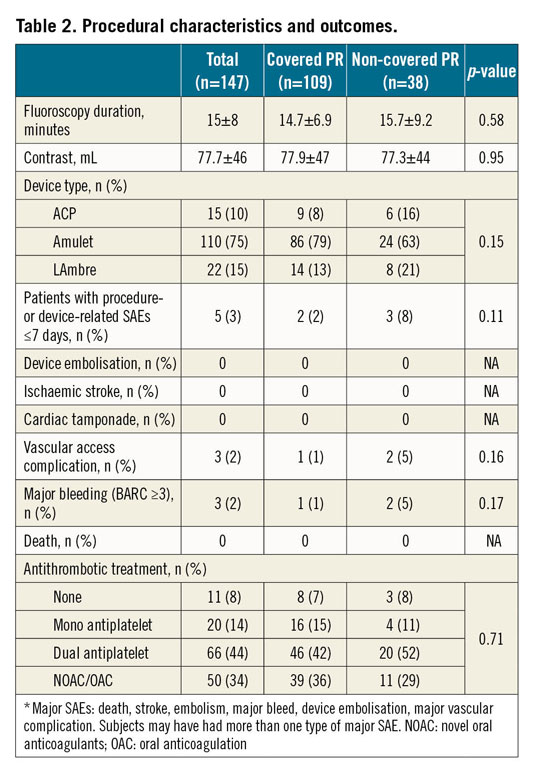
Figure 2. Percentage of patients with a covered pulmonary ridge over the period of the learning curve. Patients are presented in groups of 25.
CLINICAL AND IMAGING FOLLOW-UP
After a mean follow-up of 1.77±2.2 years, the annualised ischaemic stroke and major bleeding rates were 1.3%/year and 6.5%/year, respectively, without differences between the groups. All-cause and cardiovascular/unknown mortality was 11.7%/year and 5.4%/year, respectively, without differences between the groups (Table 3).
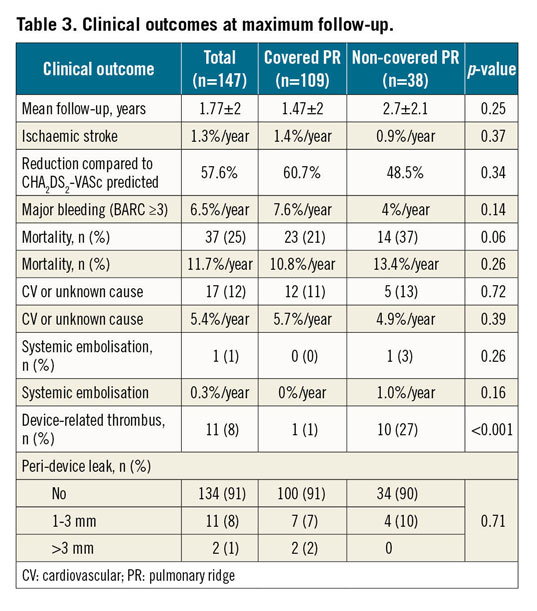
Imaging follow-up was available in 138 patients (94%). Device-related thrombosis was detected in 11 patients (7.9%). Overall, 5% of DRTs were detected ≤3 months and 2.9% between 4 and 12 months after the procedure. Figure 1 shows individualised screenshots of every single detected DRT in our series. Patients with a covered PR presented a lower incidence of DRT (1%) than those with an uncovered PR (27%); p<0.001. Most of the DRTs were located in the triangular area below the PR (Figure 1B-Figure 1L). The rate of significant PDLs (>3 mm) was 1%, without differences between the groups.
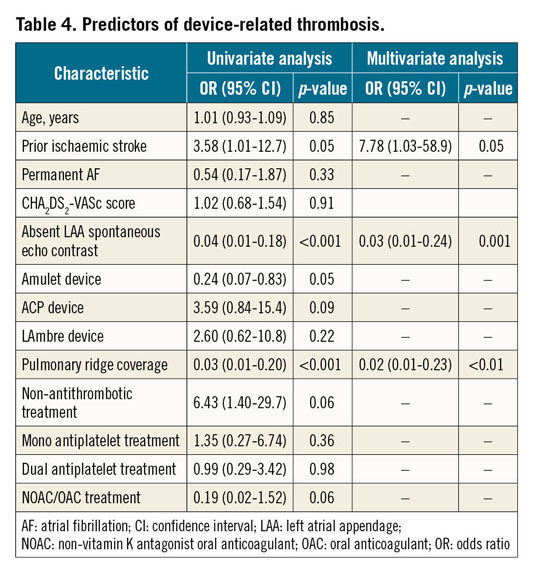
In a multivariable analysis including age, prior ischaemic stroke, permanent AF, occlusion devices, LAA spontaneous echo contrast, PR coverage and antithrombotic treatment (Table 4), the presence of PR coverage emerged as an independent predictor of DRT. Additionally, a history of previous ischaemic stroke and the presence of spontaneous echo contrast were also identified as independent factors for DRT.
Discussion
The present paper explores the impact of PR coverage after LAAO. The main findings of the study were: 1) PR coverage was associated with a lower incidence of DRT after LAAO; 2) procedural and follow-up clinical outcomes did not differ between covered PR and uncovered PR patients; 3) the percentage of cases in which the PR was covered increased with operator experience.
Patients with a covered PR presented a lower incidence of DRT after LAAO (1% vs 27%; p<0.0001). The absence of PR coverage has been suggested to be a potential risk factor for DRT in patients undergoing LAAO with the Amulet device as >80% of DRTs appeared in the area/triangle below the PR11,13. However, this is the first study reporting a significant relationship between DRT and coverage of the PR. In our cohort, the overall rate of DRT might seem high (7.9%) as compared with previous large registries with the AMPLATZER Cardiac Plug (3.2%) or the AMPLATZER Amulet (1.7%/year)1,11,19,20. Nonetheless, the observed rate was in agreement with other “real-world” large series such as the one described by Fauchier et al9, in which the global rate of DRT ranged between 5.5% and 11% in patients with available imaging at follow-up. In fact, a repeat imaging test at follow-up is one of the most important predictors of DRT incidence. Lopez-Minguez et al21 reported a DRT rate of up to 14% in patients followed with three TEEs during the first year. In our series, 36% of DRTs were detected beyond the third month after LAAO, which means that they would have been missed with just one follow-up TEE within the first three months. In this sense, a recommendation of at least two imaging tests during the first year after LAAO seems advisable in order to detect the actual DRT incidence18. Multivariable analysis confirmed that the absence of PR coverage was an independent predictor of DRT (Table 3). In addition, it also revealed the presence of spontaneous LAA echo contrast and prior ischaemic stroke as independent predictors of DRT. The role of antithrombotic therapy was also tested in the multivariable model, with a trend observed towards a higher incidence of DRT in patients without antithrombotic therapy and a lower incidence of DRTs in patients treated with oral anticoagulation (OAC)/novel oral anticoagulation (NOAC) after LAAO. In fact, low-dose NOAC seems to be an attractive alternative to provide enough DRT prevention while keeping a low risk of major bleeding as compared to dual antiplatelet therapy (DAPT) or even single antiplatelet therapy (SAPT)12,22,23. This observation seems to be an expected finding that reinforces the idea of individual antithrombotic tailoring based on the bleeding and DRT risk. In this regard, covering the PR with the disc of the device may allow less aggressive antithrombotic therapy. On the other hand, for those patients with extremely high bleeding risk in whom no antithrombotic therapy post LAAO is planned, achievement of complete PR coverage might be crucial in order to minimise the risk of DRT. Indeed, PR coverage and DRT prevention might be seen as coronary stent apposition and stent thrombosis prevention. In cases of suboptimal stent apposition, the risk of stent thrombosis would be higher, and more intensive antiplatelet therapy might be required24.
The absence of PR coverage after LAAO might generate areas of low blood velocity and flow turbulence at the level of the area below the PR13,14. Subsequently, the presence of flow turbulence and low blood velocity would explain the typical location of DRT at this level. The area between the uncovered part of the PR and the disc of the device creates a “triangular area” (Figure 1A) where all the aforementioned flow phenomena might occur. Aminian et al11 have already reported a higher incidence of DRT (>80% of the total DRT) within this “triangular area”. It is important to note that, when the disc of the device fully covers the PR, the triangular low-flow area is avoided. In our series, all DRTs were located at this level, highlighting the importance of our findings. The only patient with DRT and covered PR presented a laminar and thin DRT (Figure 1L)25. Of note, the patient was on no antithrombotic therapy after LAAO and DRT was successfully treated with SAPT. In fact, those laminar and thin DRTs have been classified as “low grade” DRT and may not require specific treatment25. From the clinical perspective, none of the patients with DRT presented any ischaemic stroke or TIA at follow-up. The only reported clinical event was a mesenteric embolism that occurred two months after detecting the DRT and while the patient was under NOAC treatment.
Another interesting finding of the present study was the increasing percentage of patients in whom the PR was covered (Figure 2). The rate of PR coverage in the first half of the cohort ranged between 60% and 70%, whereas it increased progressively up to 87% in the last 25 patients. This observation was clearly linked to the learning curve of the procedure. During the initial experience, operators might have been more focused on avoiding complications than aiming for an optimal device positioning. This means that operators probably avoided multiple recaptures or more proximal implantations in order to prevent the potential risk of LAA perforation or device embolisation. In addition, evidence in favour of PR coverage and its association with potential DRT was scarce. Nonetheless, with increasing experience and more recent DRT data, operators seemed to aim for more optimal device positioning. Additionally, 3D imaging techniques, namely CT or 3D-TEE, were used more and more, allowing a better LAA assessment and more precise device selection26. In this regard, simulations of device size/position may constitute helpful tools to ensure not only a safe but also an optimal device implantation27.
It is difficult to anticipate whether our main findings can be translated to patients with lobe-only devices such as the WATCHMAN. The rounded surface of the lobe as compared to the flat surface of the disc might generate other effects over the device. In any case, it seems reasonable to think that the absence of PR coverage might also be associated with low-flow velocity and turbulence areas regardless of the type/configuration of the device. Again, optimal coaxial and more proximal implantations would minimise the size of these areas. Further research will be necessary to prove whether our observations are also applicable to lobe-only devices.
Limitations
The present report has several limitations that should be acknowledged. This is an observational study. The clinical and TEE results were self-reported and there was no independent adjudication. Nonetheless, DRT and pulmonary ridge coverage were carefully assessed by two experienced operators. In addition, individual images of every detected DRT are provided in Figure 1B-Figure 1L.
Conclusions
The present paper explores the impact of PR coverage after LAAO with lobe and disc devices. Pulmonary ridge coverage was associated with a lower incidence of DRT after LAAO. Procedural and follow-up clinical outcomes did not differ between covered PR and uncovered PR patients. The percentage of cases in which the PR was covered increased with operator experience.
|
Impact on daily practice The present paper explores the impact of PR coverage after LAAO with lobe and disc devices. The percentage of cases in which the PR was covered increased with operator experience. Pulmonary ridge coverage was associated with a lower incidence of DRT after LAAO. |
Conflict of interest statement
X. Freixa is a proctor for Abbott Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

