Abstract
Thrombus formation on intracardiac devices remains a subject of importance, with rates in the 2-5% range. Device-related thrombus (DRT) following left atrial appendage occlusion is an area of particular concern considering its association with embolic events. DRT continues to present numerous questions, including the optimal definition, incidence, risk factors, monitoring, therapy, and clinical outcomes – all subjects of ongoing assessment. Herein, we discuss these considerations, building upon the relevant historical context and pathophysiologic insights while discussing the future considerations in this rapidly evolving field.
Introduction
Intracardiac device healing and thrombus formation is of considerable interest given the breadth of devices implanted and the subsequent finding of associated thrombus1. In a large early series of 1,000 patients where septal closure devices were used1, the average incidence of device-related thrombus (DRT) at 4 weeks post-implant was 2.0% (range 0-7.1%). There is growing interest in the complications of DRT in patients undergoing left atrial appendage occlusion (LAAO). Despite its relatively low frequency (approximately 3-5%), its association with elevated rates of stroke and systemic embolism raises continued concerns234567891011. Fundamental issues surrounding DRT relate to the diagnostic criteria used, specific imaging technologies, incidence and timing, risk factors, treatment strategies, and its cause/effect on subsequent adverse events. This article focuses on these issues.
Historical context and diagnostic strategies
DRT with LAAO was initially described in relation to the Watchman device in the first randomised controlled trial (RCT) - PROTECT AF. A transoesophageal echocardiography (TOE) core laboratory was implemented for assessment of both the initial procedural images and subsequent studies. Post-LAAO TOE studies obtained at 6-week follow-up were analysed in 485 patients12 with the intent to develop consensus echocardiographic diagnostic criteria for DRT and determine its incidence. Investigators independently scored each study as DRT present or absent. The definition of thrombus included: a filling defect that was either (1) “laminar”, if the basal length of the thrombus was greater than the height, or (2) “pedunculated”, if the height was greater than the basal length. The investigators also noted DRT position, particularly whether it covered the device connector pin site. Blinded consensus reading and differences in final diagnosis were resolved. Thrombus was identified in 5.7% of patients; in these patients, the primary efficacy events (stroke, peripheral embolism, or cardiac/unexplained death) occurred at a rate of 3.4 per 100 patient-years of follow-up. Since that initial description, TOE has been the standard modality of imaging diagnostic pre-, intra-, and post-procedure.
Another diagnostic strategy has subsequently been implemented. Cardiac computed tomography (CT) has been utilised for preprocedural assessment planning since the early implementation of LAAO1314. More recently, the role of CT has expanded to include post-procedural surveys of peri-device leaks (PDLs) and DRT. Standardised CT classification schemes for DRT have been developed which rely on documentation of hypoattenuated thickening (HAT) on the device surface cap, with HAT <3 mm defined as low grade (the clinical outcome of which is uncertain), and HAT >3 mm defined as high grade and diagnostic of DRT14.
The relative role each of these strategies may play remains unclear. Although no randomised studies directly compare the two modalities, Korsholm et al14 compared CT and TOE in 301 consecutive patients undergoing LAAO, with a blinded investigator evaluating images; 248 of the patients had imaging by CT and TOE at 8 weeks and 139 at 12 months. DRT was defined on TOE as an echo-dense mass attached to the device as per the initial PROTECT AF trial investigators. For CT, DRT included high-grade HAT (>3 mm), while low-grade HAT was abnormal but not definite. At 8 weeks, DRT was identified in 5 (2%) patients on TOE, while CT revealed 6 (2.4%) high-grade HAT and 9 (3.6%) low-grade HAT cases. At 12 months, 2 (1.4%) patients had either DRT or high-grade HAT, while 13 (9.4%) had low-grade HAT. While both imaging techniques were similar for the diagnosis of DRT, low-grade HAT was encountered more frequently than high-grade HAT. The clinical relevance of this finding will require more study. Indeed, developing standardised, modality-specific definitions for DRT following LAAO remains the focus of ongoing consensus efforts15.
Risk factors
The evaluation of factors associated with DRT spans several domains: 1) baseline characteristics; 2) anatomy; 3) specific device configuration; 4) device size and implant position; and 5) post-procedure medications (Table 1, Central illustration). Evaluation is challenged by differing definitions of DRT and the impact of incidental detection on routine imaging versus clinically driven imaging, amongst other factors. An area with relative paucity of data is the impact of the physiology of left atrial appendage (LAA) and left atrial (LA) function on subsequent thrombus formation. Data from PROTECT, PREVAIL, CAP, and CAP2 trials5 were analysed to help understand the effect of flow-related parameters, including LAA velocity, LA size, and the presence of spontaneous echo contrast (SEC). Similarly, Sedaghat et al16 suggested that reduced left ventricular ejection fraction (LVEF), SEC and reduced LAA peak emptying velocities were associated with subsequent DRT formation, supporting the role of baseline physiologic factors on DRT formation.
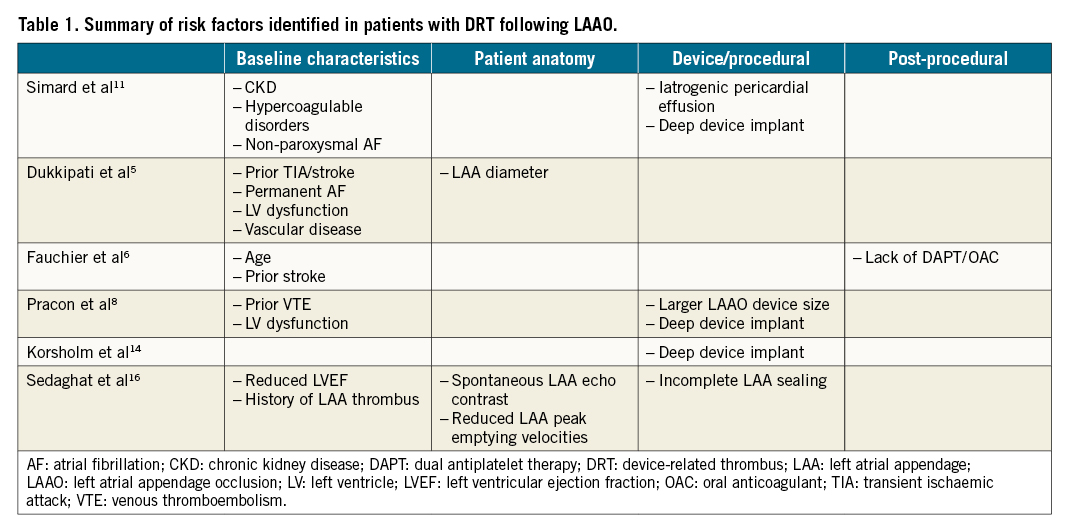
Central illustration. Factors influencing LAAO device-related thrombus formation.
The largest data set currently is the LAAO DRT Registry, a multicentre collaboration with site-reported cases from 37 worldwide institutions including 237 DRT patients and 474 controls11. Multivariable analysis identified 5 DRT risk factors: hypercoagulopathy, iatrogenic pericardial effusion, chronic renal insufficiency (CRI), implantation depth >10 mm from the pulmonary vein limbus, and non-paroxysmal atrial fibrillation. Of these, 3 might have affected procedural performance: pericardial effusion, renal insufficiency, and implantation depth >10 mm. For pericardial effusion, the procedure might have been aborted before the optimal position was obtained, while attenuated use of post-procedural anticoagulation could have also been implicated. With CRI, contrast injections may have been limited, affecting the ability to assess optimal position, while deep implantation may reflect technical procedural challenges, though the pathophysiology by which this portends DRT remains obscure. Other variables including age, sex, ventricular function, and post-LAAO medications were not predictive. The presence of 1 major risk factor (hypercoagulable state or iatrogenic pericardial effusion) or 2 minor factors (deep LAAO implant, CRI, or non-paroxysmal atrial fibrillation) was associated with double the DRT risk.
Other studies have also evaluated factors associated with DRT. Of the initial Watchman RCTs and registries totalling 1,739 patients, DRT was identified in 65 (3.74%)5 (Supplementary Table 1). DRT predictors included a history of transient ischaemic attack (TIA) or stroke, permanent atrial fibrillation, vascular disease, LAA diameter, and reduced ejection fraction (EF). Pracon et al evaluated8 102 patients who had undergone LAAO with either the Amplatzer Amulet/Cardiac Plug (Abbott; n=59) or WATCHMAN (Boston Scientific; n=43) device in a prospective registry with follow-up at 1.5, 3 to 6, and 12 months post-implantation. DRT was diagnosed in 7 patients (7.1%; 5 TOE, 2 CT) with no differences in post-implant dual antiplatelet therapy (DAPT) noted. Risk factors identified included history of thromboembolism, reduced EF, larger device size, and deeper implant position8. In the Amulet Observational Study4, in some DRT patients, the left upper pulmonary vein ridge was not covered by the disc, and the thrombus developed in the non-trabeculated area between the upper disc edge and the pulmonary vein ridge, indicating suboptimal position. Other studies have suggested that the lack of pulmonary ridge coverage has an impact on subsequent DRT in disc and lobe devices, as does lack of complete LAAO 1617. The interplay between incomplete LAAO, residual leak size, and resultant risk of DRT remains an ongoing discussion in the transcatheter and surgical LAAO literature1819. The recent Amulet IDE study demonstrated that the Amulet occluder was non-inferior for prevention of stroke/embolisation compared to the first-generation Watchman device but with improved rates of LAA occlusion and similar rates of DRT (3.3% vs 4.5%)20. The SWISS APERRO study similarly randomised patients to Amulet or Watchman, with a higher proportion (77%) of patients receiving the current-generation Watchman FLX device. This demonstrated more procedural complications but lower PDL rates with Amulet. DRT rates tended to be lower with Amulet, with CT detecting 0.9% with Amulet vs 3.0% with Watchman, while TOE reported DRT rates of 2.1% with the Amulet device vs 5.5% with the Watchman, though the distribution of Watchman 2.5 vs FLX within these groups remains undefined. The impact of post-procedural medications remains to be defined: while early Watchman studies mandated early anticoagulation, subsequent real-world registries (EWOLUTION) reported less stringent anticoagulation practices (25% on oral anticoagulants [OACs]), with DRT rates of 4.1%21. Comparatively, the Amulet Observational Study noted DRT rates of 1.7%/year, though, paradoxically, patients with DRT in this cohort were more likely to be discharged on OACs than those without DRT (DRT: 29% OAC vs no-DRT: 17.5% OAC), highlighting the ongoing knowledge gap regarding the impact of post-implant anticoagulation on DRT formation22.
Collectively, numerous risk factors have been reported, spanning patient, anatomic, technical, and post-procedural aspects. Use of a DRT risk score may help identify those populations at greater risk and refine implant and management strategies; however, these are limited by the follow-up regimens from which they are generated. Protocol-driven follow-up studies are needed to standardise these observations and provide uniform insights, particularly considering that RCT data will be less relevant to the broader populations undergoing LAAO.
Pathophysiology
The pathophysiology of DRT remains largely unknown. Potential contributing factors include both patient factors (atrial and appendage flow based on anatomy or function; coagulopathy profile) and device factors (shape, profile, fabric cap, anchor screw, and even the material used). Prior studies have assessed coagulation changes post-LAAO, with significant activation of the coagulation system signalled by elevation of prothrombin fragment 1 and 2 and thrombin-antithrombin III. In contrast, factors identifying platelet activation-soluble P-selectin and soluble CD40 ligand did not change23. Considering that prior hypercoagulable state was identified as a risk factor for DRT, clinical biomarkers of coagulopathy may also serve to identify patients at high risk for developing DRT or to identify DRT diagnosis and/or monitor resolution with therapy. The presumption remains that thrombus is the final common pathway and event, with layers of thrombus laid down over differing time intervals as part of the normal healing process.
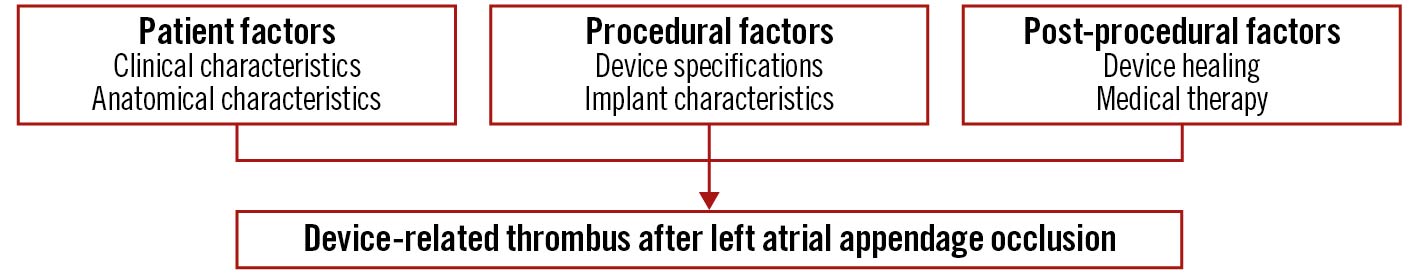
The role of endothelial cell monolayers has been emphasised for preventing thrombus formation via inhibiting smooth muscle cell proliferation and platelet adhesion24. The healing of LAAO devices (Watchman) has been studied in animal models and selected human surgical or necropsy specimens252627. In canines, at 2 days post-implant, organised thrombus covered the atrial surface and filled the gaps between the device and LAA wall. At 45 days, the fabric membrane had an organised neo-endocardial surface, covering all the exposed surfaces. Finally, at 90 days, a fibrous tissue pannus encapsulated the atrial surface of the woven fabric. This study also included 4 human autopsy hearts, with time from implant to specimen evaluation ranging from 139 to 852 days and variability in the surface coverage of the fibrin thrombus or organised neo-endocardial growth (Figure 1). The authors concluded that the healing reactions were similar between animals and humans, though more rapid in animals25. There have been other anecdotal cases. Recently, a case report was published of a Watchman device that was removed at the time of cardiac surgery; despite having been implanted 36 months previously it was not yet endothelialised, though neither DRT nor embolic events had been documented27 (Figure 2). This anecdotal case highlights the numerous factors that may be involved in the formation of DRT and device healing. This variability may be attributed to patient, procedural and device-related factors with incomplete re-endothelialisation serving as a nidus for thrombus formation.
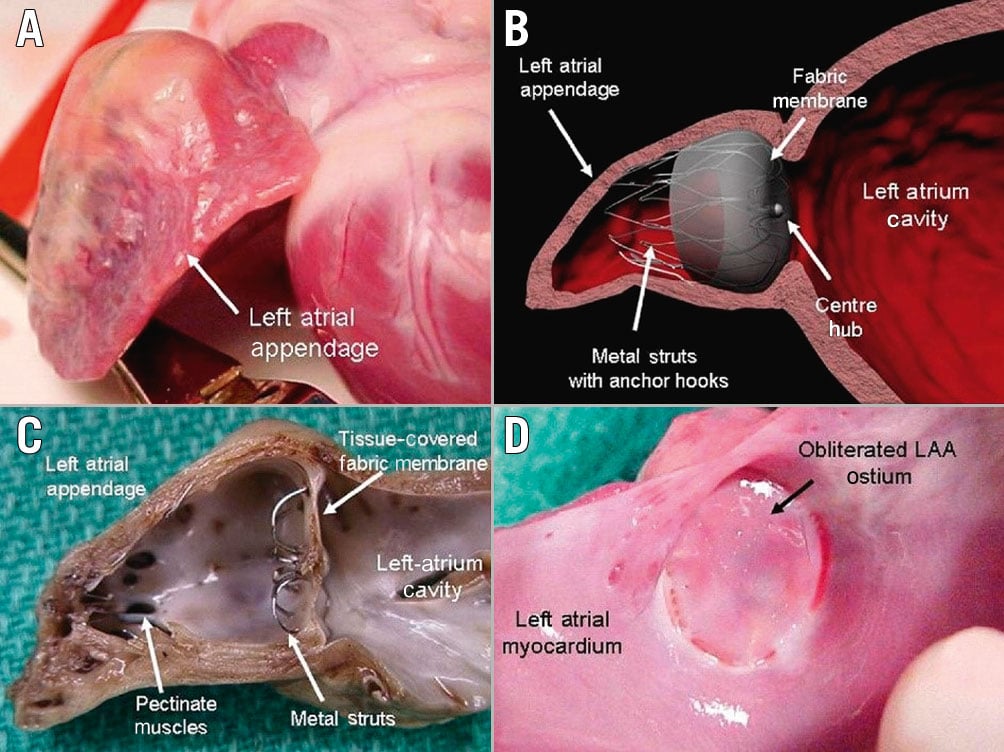
Figure 1. Left atrial appendage occlusion device healing. A. Canine heart post mortem without device implanted. B. Illustration of legacy Watchman LAAO device anchored within the LAA. C. Canine autopsy specimen 28 days post-LAAO demonstrating tissue covered left atrial device surface, with successful LAA occlusion and device re-endothelialisation noted (D). Reproduced with permission from Schwartz et al, JACC-CI 201025. LAA: left atrial appendage; LAAO: left atrial appendage occlusion
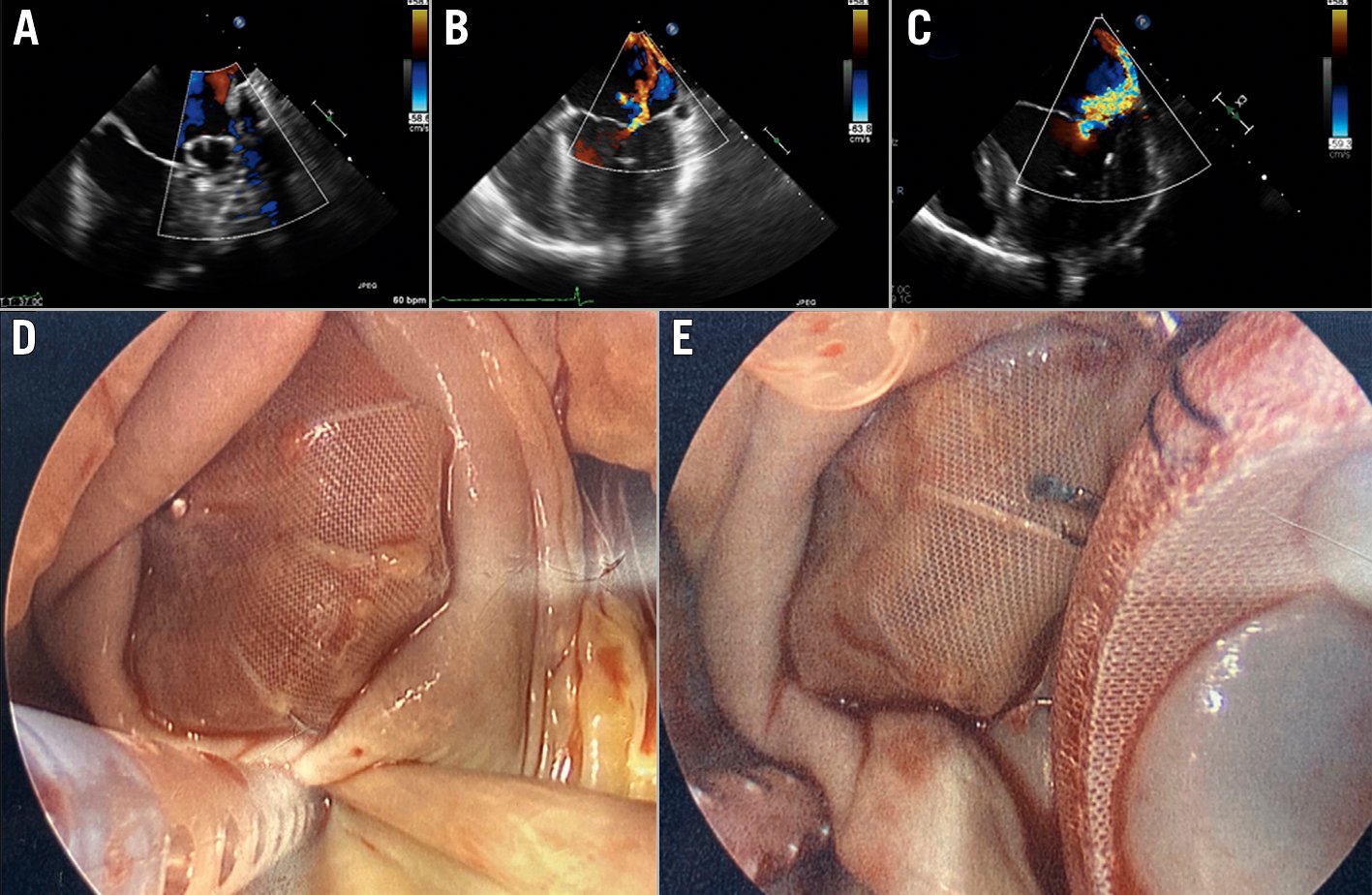
Figure 2. Delayed left atrial appendage occlusion (LAAO) device healing. Operative findings of incomplete endothelialisation in a patient who had undergone LAAO with a WATCHMAN device 37 months prior to subsequent mitral valve replacement. A. Post-Watchman device implantation TOE at 12 months showing a well-seated Watchman device without any peri-device leak or thrombus; B. Pre-Watchman device implantation TOE showing a moderate mitral regurgitation with eccentrically directed jet; C. Pre-mitral valve replacement TOE showing a severe mitral regurgitation with eccentrically directed jet; D, E. A failure of complete endothelialisation of the Watchman device at 37 months post-implantation. Reproduced with permission from Batnyam et al, JACC: Case Reports 202127. TOE: transoesophageal echocardiogram
Other data of healing have become available with both the Watchman device and the Amplatzer Cardiac Plug (ACP)26, assessing 6 canine and 19 post mortem hearts. In the Watchman canine specimens, there was complete coverage of the atrial surface of the device as well as the connector screw hub by neo-endocardial tissue at 28 days. In contrast, at 28 days with the ACP, the upper edge of the disc impinged on the lateral ridge between the LAA ostium and the left superior pulmonary vein, and only a small portion of the disc was covered by neo-endocardial tissue, without significant coverage of the inferior disc edge. Overall, the Watchman device was associated with a greater extent of healing, with less fibrin deposition and inflammation compared with the ACP device.
Specific device structural issues may also affect DRT; the bare and protuberant connector pin has been highlighted as a nidus for thrombus. Whether that pin is endothelialised or not, or whether flow patterns around it may contribute to DRT, is uncertain. CT modelling of flow dynamics has shown some signs of identifying high-risk scenarios for DRT prediction following LAAO2829. However, the complex interplay between device endothelialisation and DRT remains incompletely understood, with numerous reports describing incomplete endothelialisation at long-term follow-up that, paradoxically, still do not demonstrate marked DRT formation30313233. The most recent device, the FLX, has minimised the connector pin protuberance, and the current evidence at 1 and 2 years based on 400 consecutive US pilot patients has identified a DRT incidence of only 1.7%34; a promising early finding that will require ongoing study. At present, two randomised studies are assessing direct-acting oral anticoagulants versus DAPT following LAAO for 8-week (ANDES trial, ClinicalTrials.gov: NCT03568890) or 12-week durations (ADALA trial, EudraCT 2018-001013-32).
These and other studies may be very challenging, given the large number of patients that will be required considering the low incidence of DRT, and multiple factors impacting DRT formation. With the ACP, and now the Amulet device, the importance of disc coverage and variable healing patterns remains to be discerned. Device-specific refinements including coatings to facilitate endothelialisation may provide some improvements.
Incidence
DRT incidence has varied across several studies (Supplementary Table 1). In the largest composite of Watchman studies, which included 3,181 patients from 4 datasets, the incidence ranged from 3.7% to 5.5%5. Selection bias challenges all these findings as there are no well-controlled studies of consecutive large series of patients who have undergone routine surveillance studies. Amplatzer devices may demonstrate greater variability, with DRT rates ranging from 16.7%16 to 3.2%10 without association with embolic events demonstrated. Later analysis of the Global Amplatzer Observational Study of 1,078 patients identified 18 DRTs in 17 patients (1.7% per year)4. Here, DRT was associated with increased TIA/stroke rates, though most of the DRT patients (82%) did not experience a clinical neurological event but may have had subclinical emboli. A direct scientifically controlled head-to-head comparative analysis of the specific incidence of DRT with either the Watchman or Amplatzer device is not available. Alkhouli et al2 performed a meta-analysis of 66 studies and 12,033 patients with pooled DRT incidence of 3.8% and no difference between the Amplatzer and Watchman devices. DRT diagnosis timing varied with differing patterns observed, but a general trend of a bimodal distribution with both early and late DRTs was observed211 (Figure 3). Certainly, the timing of diagnosis is inherently linked to the imaging regimen pursued, with the empirical concept of early and late imaging appearing justified, and clinically-driven imaging (i.e., following an embolic event) most likely leading to further DRT detection. Indeed, the frequency with which imaging is performed will invariably be associated with the rate of DRT detected 35. Implementing unbiased standardised surveillance protocols and core laboratory evaluation of cases based upon specific modalities and definitions would be beneficial to obtain insights into DRT timing, device-specific considerations, optimal follow-up regimens, and into DRT persistence or recurrence. Going forward, particularly with studies now comparing LAAO to medical therapy, the considerations of standardised LAA imaging protocols in both device and medical therapy cohorts will be of similar importance.
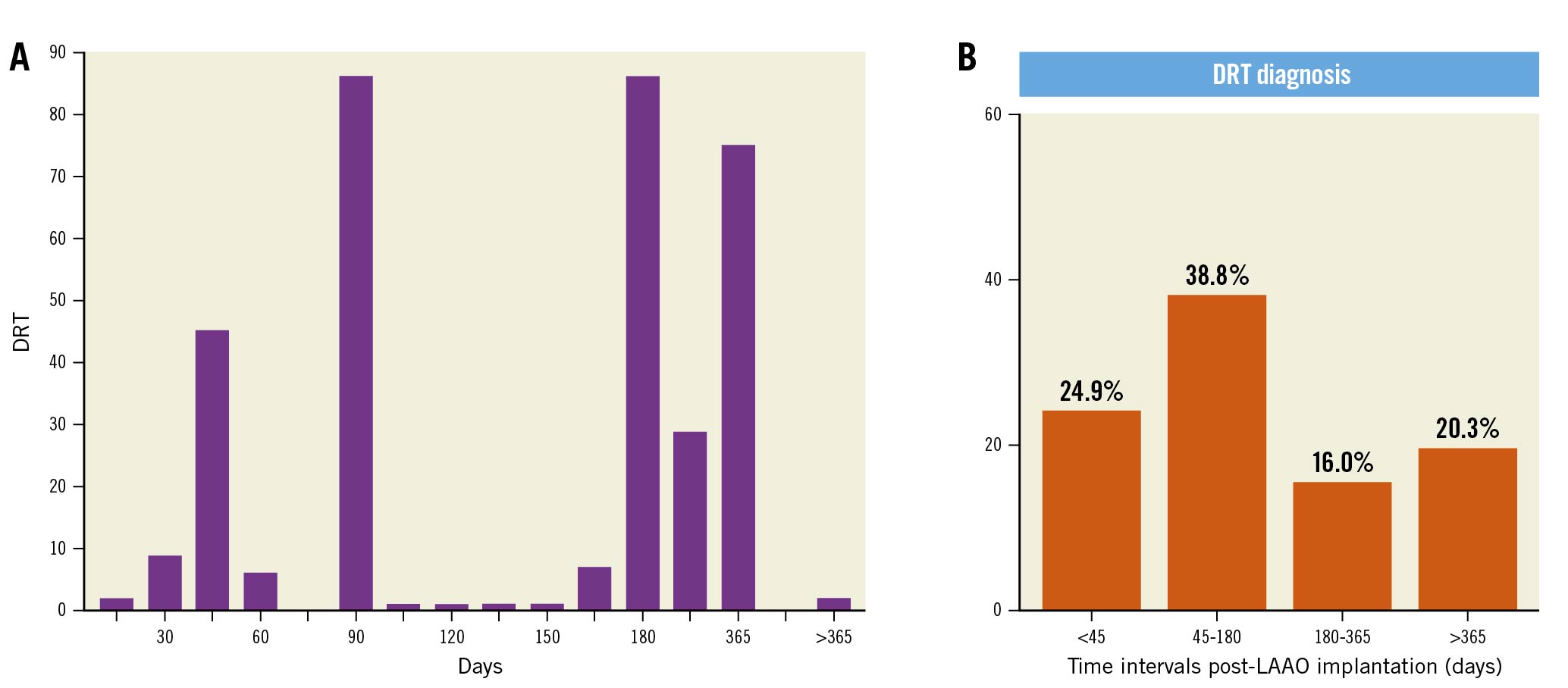
Figure 3. Temporal distribution of DRT diagnosis. Varying patterns of DRT diagnosis intervals highlighting the importance of surveillance imaging. Reproduced with permission from Alkhouli et al JACC Clin Elect 20182 (A) and Simard et al JACC 202111 (B). DRT: device-related thrombus; LAAO: left atrial appendage occlusion
Treatment
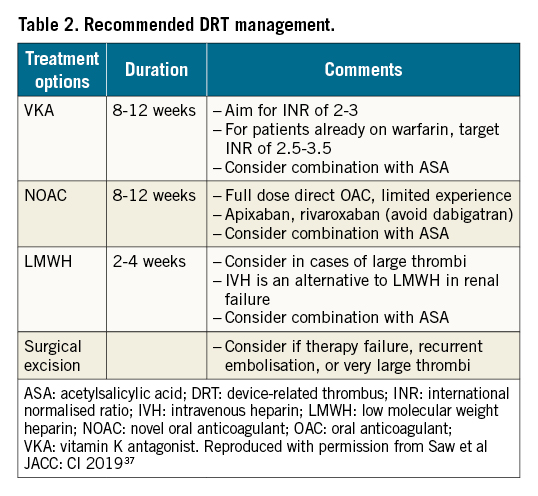
The optimal treatment of DRT is an area of ongoing debate282136 37 38. Recommendations for the initial treatment of newly diagnosed DRT vary36 (Table 2). Although literature suggests that anticoagulation re-initiation is effective in resolving DRT in >90% of patients, concerns remain about (1) the candidacy of these patients to resume anticoagulation, (2) the duration of treatment needed, and (3) the risk of DRT recurrence upon cessation of anticoagulation. This latter issue is particularly concerning given that up to 50% of patients with DRT may suffer a recurrence once the anticoagulation course for the index DRT is completed37. DRT treatment recommendations vary considerably, with incidental versus clinically-driven diagnoses contributing to some extent, while specific DRT patterns may require different care approaches. Re-initiation of an OAC is often employed and continued for 2-3 months with important implications. Given that many patients are referred for LAAO because of relative or even “absolute” contraindications for anticoagulants, the identification of DRT, and subsequent requirement for OAC, may have significant implications, particularly for gastrointestinal (GI) bleeding 39. Accordingly, if DRT is identified and OAC treatment initiated, the treatment duration should be limited, with serial imaging to assess resolution. Alternative strategies in high-risk patients include a short 2-3 week course of low molecular weight heparin (LMWH) or addition of a second antiplatelet agent, in addition to GI prophylaxis. DRT treatment, of which there is considerable variability in practice patterns reported, leads to resolution in 75% of cases, with the remainder reflecting DRT persistence or recurrence – factors that remain to be discerned11.
Clinical outcomes
The association of DRT with clinical events has been a focus of great interest. Observer bias is a major limitation of studies that do not have standardised protocols, with clinically-driven imaging biasing event rates, highlighting the need for dedicated registries. In the initial Watchman RCTs5, during follow-up, 16/65 (25%) patients with DRT experienced an ischaemic stroke or systemic embolism compared with 114/1,674 (6.8%) patients without a DRT (p<0.001). Both unadjusted and adjusted rates of ischaemic stroke, as well as all stroke and systemic embolism, were higher in DRT patients, without a difference in mortality (Supplementary Table 2)5. Strokes in DRT patients accounted for approximately 10% of all strokes, but 75% of patients with DRT did not develop a stroke (although this needs to be substantiated with longer-term follow-up) (Figure 4). In the EWOLUTION European trial of 1,020 patients treated with a Watchman device, despite an incidence of 4.1% of DRT, there was no difference in cardiac event rates of death, ischaemic stroke or the composite of death/ischaemic stroke/TIA21. With the Amplatzer Cardiac Plug, there was also heterogeneity in a study of 1,078 patients, in which DRT was associated with a greater risk of ischaemic stroke or TIA compared with those without DRT (HR 5.27, 95% CI: 1.58-17.55; p=0.007)4. Conversely, Saw et al10 did not note an association between DRT and adverse cardiac events. In the meta-analysis of 66 studies2, the pooled incidence of ischaemic events of patients with and those without DRT was 13.2% (with) and 3.8% (without) (95% CI: 3.66-7.59; p<0.001). Sensitivity analysis including only randomised trials and prospective multicentre registries demonstrated a DRT incidence of 3.7% and an association with ischaemic events (13.5% [with] versus 4.4% [without DRT]) (95% CI: 2.77-6.22; p<0.001).
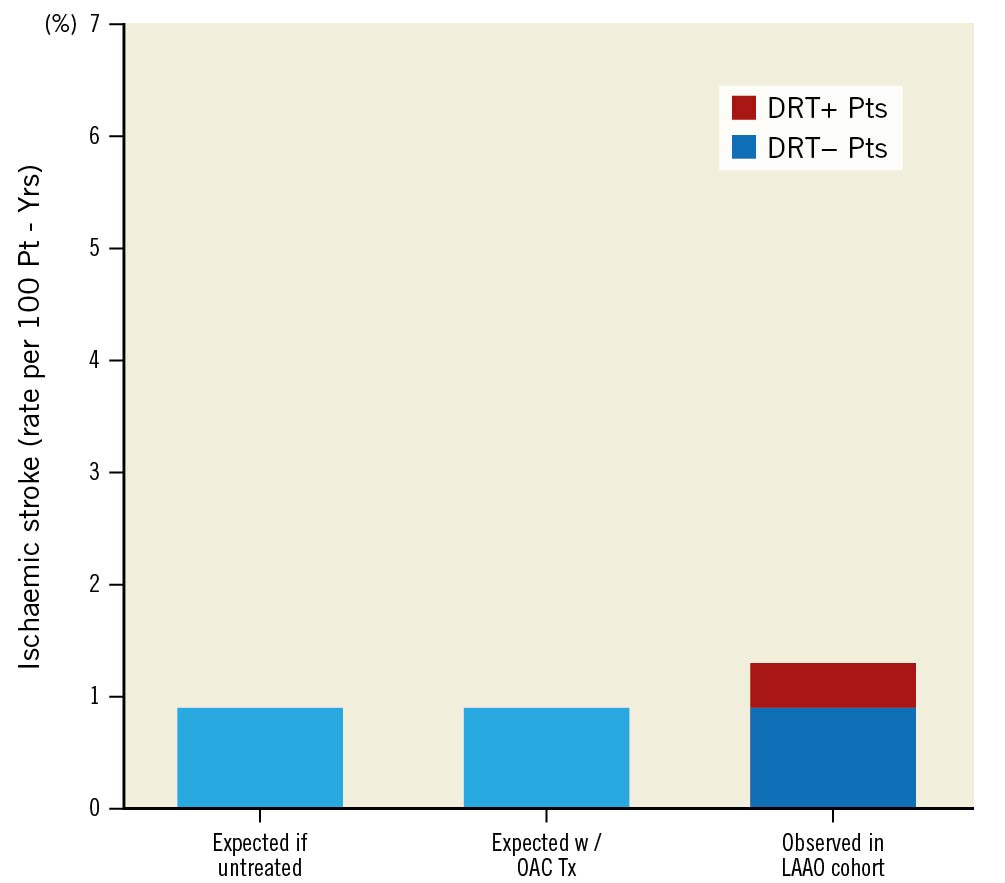
Figure 4. Comparison of ischaemic stroke rates – expected versus observed in LAAO cohort. Patients with device-related thrombus (DRT) represented 10.2% of all ischaemic strokes noted in follow-up. Comparison to expected stroke rates calculated based on CHA2DS2-VASc score of the cohort if untreated or following oral anticoagulant therapy. Reproduced with permission from Dukkipati et al Circulation 20185. LAAO: left atrial appendage occlusion; OAC: oral anticoagulant; Tx: treatment/therapy
Discussion and future directions
To date, only 3 LAAO RCTs have been completed, with several more in progress comparing LAAO to oral anticoagulants (Supplementary Table 3). They will evaluate specific clinical scenarios (i.e., Watchman FLX and Amulet compared with direct-acting oral anticoagulants [DOACs], patients undergoing atrial fibrillation ablation, and patients who have a contraindication to OACs) and will answer many important questions. Continued data accumulation in multiple registries supports the safety and efficacy of LAAO. DRT has an incidence of 3-5% and potential association with elevated embolic risk. When identified, it leads to considerable patient and physician concern, with varying therapeutic regimens and follow-up protocols pursued. Taken together, DRT has been the focus of intense interest.
Improving our understanding of the prevention and treatment of DRT will be challenged by the multiple contributing factors and low incidence rate. Alternatively, new devices with improved coatings and recessed pin connectors may be employed in larger post-approval studies. Polymer coatings have been widely used with coronary stents previously and have been widely exposed to regulatory bodies. Accordingly, addition of these polymers to the surface of an LAAO device, as proposed in the novel Watchman FLX good laboratory practice (GLP), may not be viewed by the United States Food and Drug Administration (FDA) as a significant change in the device (Figure 5). If so, this would facilitate implementation of post-approval studies to ensure no adverse clinical outcomes while avoiding the need for larger studies. In theory, these could be incorporated into the larger multicentre RCTs such as CHAMPION or CATALYST, with a subset of patients that could be enrolled in a surveillance study using routine CT or TOE to document the incidence of DRT compared with the information from the larger meta-analysis, to confirm at least non-inferiority, and possibly, superiority. Moreover, ongoing trials of medical versus device therapy for stroke prevention raise the question of the role for standardised LAA imaging in both cohorts to empirically assess the rates of intracardiac thrombus.
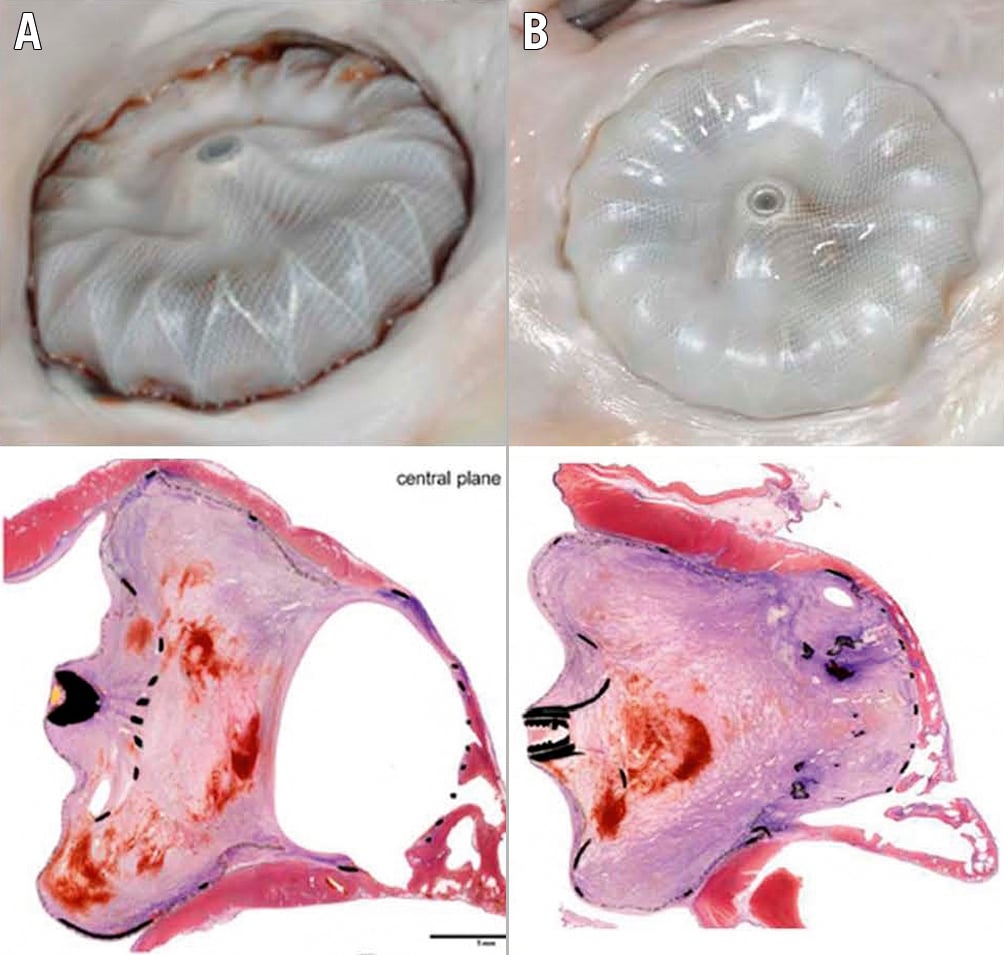
Figure 5. Novel Watchman FLX GLP polymeric device coating. Demonstration of device healing with polymeric coatings of atrial aspect of device intended to facilitate endothelialisation with preliminary canine post mortem assessments at 45 days (A) and 90 days (B) demonstrating excellent endothelialisation. Image provided courtesy of Boston Scientific. © 2021 Boston Scientific Corporation or its affiliates. All rights reserved.
Conclusions
DRT following LAAO is a significant clinical safety issue. Although relatively infrequent at 3-5%, it is often associated with an increased risk of stroke/systemic embolisation events. Information on the issue of association versus causation is not available. Notable concerns are related to the lack of standardised imaging protocols that would shed significant light on the incidence, risk factors, and association between various imaging abnormalities and subsequent clinical events. This information would enable optimisation of strategies to prevent and treat DRT. The implementation of a dedicated registry as part of one of the large RCTs, or a separate smaller focused registry, would help to guide the field toward resolution of the issues involved.
Conflict of interest statement
T. Simard received consulting fees from Boston Scientific. B. Hibbert participates in clinical trials with Boston Scientific, Abbott, Edwards Lifesciences, and NXT. M. Alkhouli is on the CHAMPION trial steering committee and advisory boards for Boston Scientific and Philips, and declares consulting fees from Boston Scientific and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

