
Abstract
Aims: The use of large-diameter sheaths carries the risk of significant vascular and bleeding complications after transfemoral transcatheter aortic valve implantation (TAVI). In this analysis, we sought to assess the impact of a modified femoral artery puncture technique using digital subtraction angiography (DSA) and road mapping during transfemoral TAVI on periprocedural vascular and bleeding events.
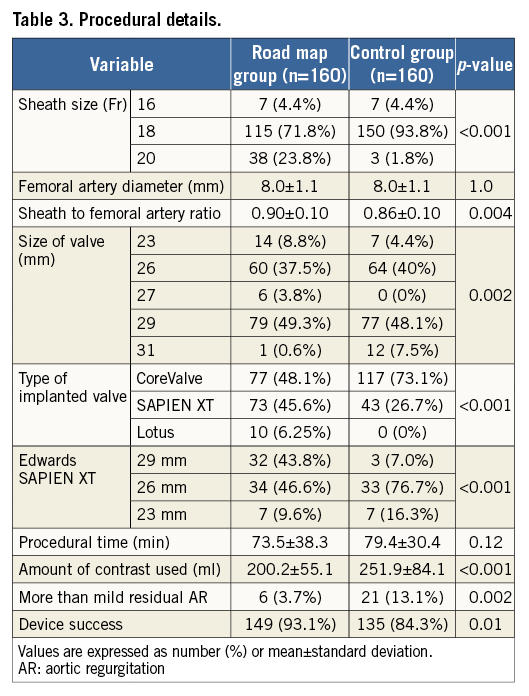
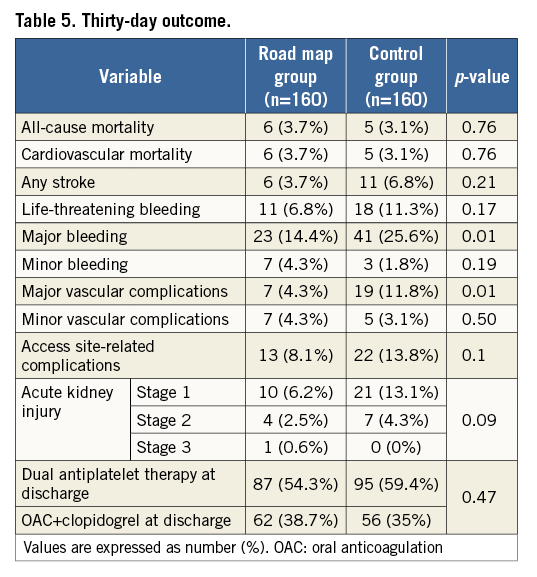
Methods and results: This is a retrospective analysis of transfemoral TAVI patients included in a prospective institutional database. The modified femoral artery puncture technique using DSA-derived road mapping guidance was introduced in October 2012. Before the introduction of this technique, vascular puncture was acquired based on an integration of angiographic data, the bony iliofemoral landmarks and a radiopaque object. Consecutive patients who underwent TAVI with the road mapping technique (RM group, n=160) were compared with consecutive patients who underwent TAVI without road mapping (control group, n=160) prior to its introduction. A standardised strategy of periprocedural anticoagulation was adopted in both groups as well as the use of a single suture-based closure device. All endpoints were defined according to the VARC-2 criteria for event definition. The mean age in the RM group was 80±7.7 years compared to 81±5.9 years in the control group (p=0.19), and females were equally distributed between both groups (63.1% vs. 58.1%, p=0.36). The baseline logistic EuroSCORE was 20.7±14.4% vs. 24.9±15.2% in the RM and control group, respectively (p=0.01). Notably, sheath size was significantly larger in the RM compared to the control group due to the more frequent use of the 20 Fr sheath (23.8% vs. 1.8%, p<0.001, respectively) associated with the more frequent implantation of the 29 mm Edwards SAPIEN XT valve in the RM group (43.8% vs. 7%, respectively, p<0.001). Despite the latter finding, both major vascular complications and major bleeding at 30 days were significantly lower in the RM group compared to the control group (4.3% vs. 11.8%, p=0.01, and 14.4% vs. 25.6%, p=0.01). An analysis limited to access site-related complications also revealed lower events in the road map group but did not reach statistical significance (8.1% vs. 13.8%, p=0.1). Other forms of vascular and bleeding complications as well as all-cause mortality were comparable in both groups.
Conclusions: A modified femoral artery puncture technique using DSA and road mapping was associated with a reduction in major vascular and bleeding complications after transfemoral TAVI, and provides a simple and effective strategy for potentially improving patient outcomes.
Introduction
Access-site complications are an important cause of morbidity and mortality in patients treated with transfemoral transcatheter aortic valve implantation (TAVI)1-3. The use of large-diameter sheaths in an elderly patient population carries the risk of significant vascular and bleeding events. In early large registries and clinical trials, transfemoral arterial access was associated with vascular complications in 11-18% of procedures1,4. In a weighted meta-analysis of 3,519 patients from 16 studies, the pooled estimate rate of vascular complications defined according to the Valve Academic Research Consortium (VARC) was 18.8%5.
Several predictors of vascular complications have been previously identified, including early site and operator experience, moderate/severe iliofemoral artery calcification, minimal artery diameter and a sheath to femoral artery ratio of more than 1.056-8.
An appropriate vascular access with a true anterior puncture in a disease-free segment of the common femoral artery is essential for limiting these complications and could potentially improve overall clinical outcomes. In this analysis, we sought to assess the impact of a modified femoral artery puncture technique using digital subtraction angiography (DSA) and road mapping on periprocedural vascular and bleeding events after transfemoral TAVI.
Methods
PATIENT POPULATION
Our institutional TAVI programme started in September 2007. All patients are prospectively included in a dedicated institutional database and are followed up at regular predefined time intervals (30 days, six months, one year and yearly thereafter). Until July 2014, 460 consecutive patients underwent transfemoral TAVI at our institution. The modified femoral artery puncture technique using road mapping guidance was introduced in October 2012. Briefly, a fluoroscopic sequence is acquired during contrast injection (10 ml) from a contralaterally inserted pigtail catheter positioned at the aortic bifurcation. This “road map mask” is then subtracted to produce a real-time fluoroscopic image overlaid on a static image of the iliofemoral vessels (Figure 1). Before introduction of this technique, vascular puncture was acquired based on an integration of angiographic data, the bony iliofemoral landmarks and a radiopaque object (Figure 2). Consecutive patients who underwent TAVI with implementation of the road mapping technique (RM group, n=160) were compared with consecutive patients who underwent TAVI without the road mapping technique (control group, n=160) prior to its introduction. Patients who underwent TAVI earlier (n=80) were excluded from this analysis in an attempt to reduce the impact of the learning curve on measured outcomes. Moreover, patients who received the new-generation balloon-expandable SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) utilising a smaller introducer sheath (n=60) were also excluded. TAVI was only performed for patients who were deemed inoperable or at high surgical risk for conventional surgery based on high morbidity and mortality scores and/or other comorbidities after discussing the patient’s data within a multidisciplinary Heart Team, as suggested in the current recommendations9. All patients provided written informed consent to undergo the procedure and for analysis of their anonymised data. Data collection was conducted in accordance with the Declaration of Helsinki and was approved by an independent ethics committee as per local regulations.
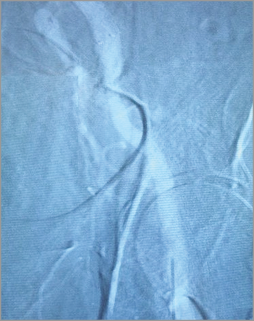
Figure 1. “Road map mask” subtracted to produce a real-time fluoroscopic image overlaid on a static image of the iliofemoral vessels.
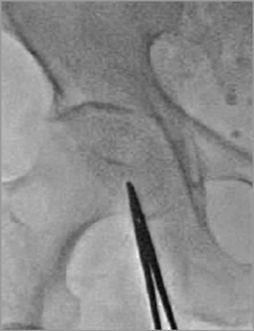
Figure 2. Previous puncture technique: integration of angiographic data, the bony iliofemoral landmarks and a radiopaque object.
PRE-INTERVENTIONAL VASCULAR ACCESS-SITE ASSESSMENT
We assessed the vascular access site using a combination of imaging modalities: invasive peripheral angiography (using 15-20 ml of contrast) during pre-procedural coronary angiography, multidetector computed tomography (MDCT) and duplex ultrasonography. The appropriate access site was chosen depending on the minimal femoral and iliac arteries’ luminal diameter, taking into consideration the presence of tortuosity, calcifications and the location of the common femoral artery bifurcation. The sheath to femoral artery ratio was calculated from the femoral artery diameters obtained from MDCT and the pre-specified inner sheath size dimensions.
PERIPROCEDURAL MANAGEMENT
All but four procedures (98.7%) were performed under analgosedation (without endotracheal intubation) using fluoroscopic guidance. A standardised vascular access closure strategy was adopted in all patients, using a single suture-based closure device (Perclose ProGlide®; Abbott Vascular, Santa Clara, CA, USA). This is a 6 Fr haemostatic device consisting of a monofilament suture and a preformed knot. After puncture of the common femoral artery, a standard 9 Fr sheath is used for predilatation, then the closure device is deployed prior to insertion of the large-bore introducer sheath. At the end of the procedure, the preloaded sutures are tied while still leaving a 0.035 inch guidewire in the femoral artery. A second ProGlide device could still be introduced over the wire if the suture is disrupted during tightening of the knot or if excessive bleeding occurs. After primary assessment of haemostasis, the guidewire is removed, the knot is further tightened and manual compression is applied for 5-10 minutes. Crossover angiography is performed thereafter (10 ml) for post-procedural evaluation of the access vessel.
Periprocedural anticoagulation was achieved with unfractionated heparin according to the activated clotting time (250-300 seconds). Patients under oral anticoagulation (OAC) therapy did not undergo TAVI unless the international normalised ratio (INR) was ≤2. Post-procedural antiplatelet and OAC therapy, if indicated, were also standardised. Patients received either dual antiplatelet therapy (aspirin and clopidogrel) for three months post TAVI followed by a single antiplatelet thereafter, or OAC therapy when indicated and clopidogrel for three months.
The following sheaths were used: 16/18/20 Fr expandable Edwards E-sheaths for the balloon-expandable SAPIEN XT valve, 18 Fr Ultimum™ sheath (St. Jude Medical, St. Paul, MN, USA) for the self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA), and the 18/20 Fr Lotus™ introducer sheaths for the mechanically expanding Lotus™ valve (Boston Scientific, Marlborough, MA, USA).
TREATMENT OF VASCULAR COMPLICATIONS
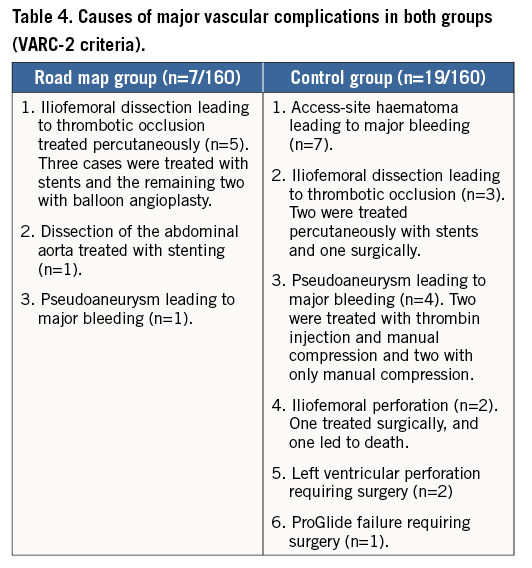
Treatment of vascular complications was left to the operators’ discretion. Significant iliofemoral dissections, thrombotic occlusions and stenoses were treated with conventional balloon angioplasty or with self-expanding stents. Perforations and/or vessel ruptures, insufficiently managed with manual compression and/or interventional techniques, were treated surgically.
ENDPOINTS
All procedural and 30-day endpoints were classified according to the Valve Academic Research Consortium (VARC)-2 definitions10. In accordance with VARC-2, vascular complications were divided into major and minor ones, and bleeding events were divided into life-threatening, major and minor bleeding10.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean values±standard deviation and were analysed with the Student’s t-test or Mann-Whitney test, as appropriate. Discrete variables are presented as counts and percentages and were analysed by Pearson’s chi-square or Fisher’s exact test, as appropriate. The level of statistical significance was set at p<0.05. Statistical analysis was performed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
BASELINE CHARACTERISTICS
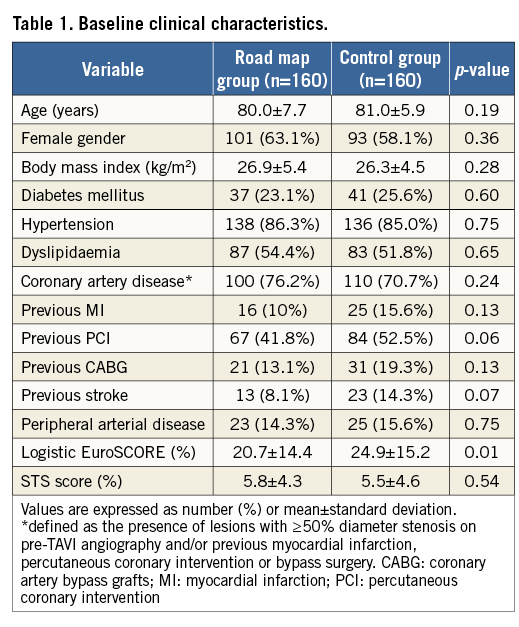
The mean age in the RM group was 80.0±7.7 years compared to 81.1±5.9 years in the control group (p=0.19), and females were equally distributed between both groups (63.1% vs. 58.1%, p=0.36, respectively). The prevalence of hypertension, diabetes mellitus, coronary and peripheral arterial disease was also comparable in both groups. Although the baseline STS score did not differ significantly between both groups (5.8±4.3 vs. 5.5±4.6, p=0.54), the baseline logistic EuroSCORE was significantly lower in the RM group than in the control group (20.7±14.4% vs. 24.9±15.2%, p=0.01). Baseline clinical characteristics are shown in Table 1.
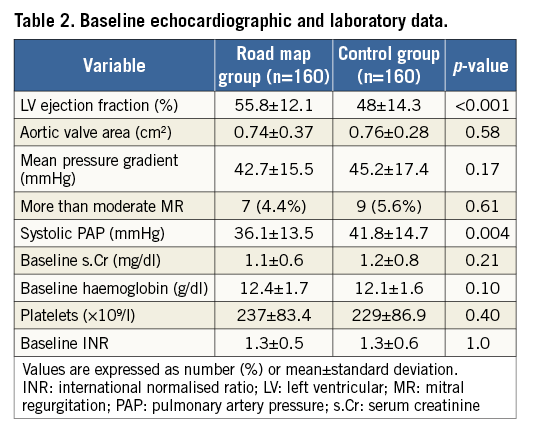
Mean left ventricular ejection fraction (EF) was higher in the RM group compared to the control group (55.8±12.1% vs. 48±14.3%, p=<0.001), but the mean aortic pressure gradient (42.7±15.5 vs. 45.2±17.4 mmHg, p=0.17) and the aortic valve area (0.74±0.37 vs. 0.76±0.28 cm2, p=0.58) were comparable. Of note, the systolic pulmonary artery pressure was lower in the RM compared to the control group (36.1±13.5 vs. 41.8±14.7 mmHg, p=0.004). No differences were noticed regarding the laboratory parameters such as baseline haemoglobin level, platelet count and the international normalised ratio (INR) between both groups. Baseline echocardiographic and laboratory data are shown in Table 2.
PROCEDURAL DETAILS
Procedural details of both groups are shown in Table 3. Three different valve prostheses were implanted during the study period. The self-expanding CoreValve was more frequently implanted in the control group (73.1% vs. 48.1%), the balloon-expandable Edwards SAPIEN XT valve (Edwards Lifesciences) was more frequently implanted in the RM group (45.6% vs. 26.7%), whereas the Lotus valve was only implanted in the RM group (6.25% vs. 0%). Overall device success was higher in the RM compared to the control group (93.1% vs. 84.3%, p=0.01), which was mainly driven by a lower rate of more-than-mild residual AR (3.7% vs. 13.1%, p=0.002). The sheath size was significantly larger in the RM group than in the control group due to the more frequent use of the 20 Fr sheath (23.8% vs. 1.8%, p<0.001) associated with the more frequent implantation of the 29 mm Edwards SAPIEN XT valve in the RM group (43.8% vs. 7%, p<0.001). As the mean common femoral artery diameter was identical in both groups, this led to a higher sheath to femoral artery ratio in the RM group compared to the control group (0.90±0.1 vs. 0.86±0.1, p=0.004). The Perclose ProGlide closure device was successfully predeployed in all patients, and only three patients had a percutaneous closure device failure (one from the RM group necessitating prolonged manual compression and two from the control group, one managed with prolonged manual compression and the other eventually requiring surgical repair, p=0.56). In addition, two closure devices were required to seal the puncture site successfully in four patients (two from each group). Of note, contrast media utilisation was significantly lower in the RM compared to the control group (200.2±55.1 vs. 251.9±84.1 ml, p<0.001), but the rate of acute kidney injury following TAVI was only numerically lower but not statistically significant (p=0.09).
THIRTY-DAY OUTCOME
Clinical follow-up at 30 days was achieved in all patients (100%). At 30 days, major vascular complications and major bleeding were both significantly lower in the RM group compared to the control group (4.3% vs. 11.8%, p=0.01, and 14.4% vs. 25.6%, p=0.01). Six of the seven major vascular complications that occurred in the RM group were managed interventionally (surgical repair was needed in one patient). On the other hand, five surgical repairs and four interventional procedures were needed in the control group. An analysis limited to access site-related complications also revealed lower events in the RM group but this did not reach statistical significance (8.1% vs. 13.8%, p=0.1). The road map technique was significantly associated with lower major vascular and bleeding complications in patients treated with the balloon-expandable SAPIEN XT valve (1.4% vs. 11.6%, p=0.01, and 10.9% vs. 30.2%, p=0.009, respectively), and numerically lower complication rates in patients treated with the self-expanding CoreValve (7.8% vs. 11.9%, p=0.35, and 18.2% vs. 23.9%, p=0.34, respectively), with no significant interaction with valve type. Table 4 lists the causes of major vascular complications in both groups. Other forms of vascular and bleeding complications as well as all-cause mortality and stroke were comparable in both groups (Table 5). There was no significant difference in the prescribed antithrombotic treatment regimen in both groups (Table 5).
Discussion
To the best of our knowledge, this study is the first to report systematically on a modified femoral artery puncture technique for transfemoral TAVI using digital subtraction angiography and road mapping. The most important findings of this study can be summarised as follows: (i) the modified puncture technique was associated with a reduction in major vascular and bleeding complications 30 days after TAVI, and (ii) this reduction occurred despite the use of larger sheath sizes and in the setting of vascular closure with, predominantly, a single suture-based closure device.
Vascular events are still among the most frequent complications after TAVI. Toggweiler et al reported an incidence ranging between 1.9% and 17.3%11. In a recent review of the literature (22 studies), the incidence in more than 12,000 patients was reported to be 5% (range between 2% and 28%)12. Using the standardised VARC criteria for event definition, a weighted meta-analysis of 3,519 patients from 16 studies has reported a pooled estimate rate of vascular complications of 18.8%5. These events have been associated with morbidity, reduced quality of life, increased costs and short-term mortality2,3,6,13.
Bleeding complications are also frequently reported after TAVI. In a meta-analysis published by Généreux et al, the pooled estimate of all bleeding complications was 41.4% (life-threatening bleeding [LTB] 15.6% and major bleeding 22.3%)5. In the PRAGMATIC PLUS initiative, blood transfusion was associated with increased mortality at one year and increased risk of major stroke and acute kidney injury14. Recently, Moretti et al reported that periprocedural bleeding after TAVI was frequent (LTB 18% and major bleeding 16%) and associated with an increased mortality after 30 days but not after midterm follow-up15.
In the present study, the use of the road mapping technique while puncturing the femoral artery reduced both major vascular complications and major bleeding events 30 days after TAVI. Of importance, these results were achieved despite a significantly larger sheath size and a higher sheath to femoral artery ratio in the RM group compared to the control group, but both were lower than the ratio defined by Hayashida et al (1.05) to predict the occurrence of major vascular complications6.
There are several techniques described to puncture the femoral artery. A surgical cut-down to expose the iliofemoral artery was initially performed when large diameter sheaths (22 Fr to 24 Fr) were used16. This has the advantage of allowing the visualisation and selection of the ideal puncture site and control of the artery above and below the puncture. With the introduction of the percutaneous transfemoral access using suture-mediated closure devices, it was necessary to obtain a precise centralised anterior wall puncture of the common femoral artery (CFA). An ideal “landing zone” may be defined as vascular entry above the femoral bifurcation and below an upper margin, conservatively defined as several centimetres below the inferior excursion of the inferior epigastric artery (IEA). The IEA descends to, but does not cross, distal to the inguinal ligament; thus, entry above the lowest point of the course of this vessel, which typically then turns cranial to supply the epigastrium, can be used to define an unequivocally high puncture. In general, the level of the mid third of the femoral head is an approximation of the level of puncture for the CFA17. A too high puncture is not well compressible and may injure the IEA, leading to unnoticed bleeding and a retroperitoneal haematoma. On the other hand, a too low puncture, in the superficial femoral artery, profunda femoris artery or the common femoral bifurcation is not recommended, as the risk of local complications such as vessel laceration, perforation, pseudoaneurysm, arteriovenous fistula, thrombosis or excessive bleeding is high18.
There are several ways to achieve such an accurate vascular puncture percutaneously. Ultrasonography can be used to visualise the artery and guide the puncture. Alternatively, a radiopaque instrument or a catheter positioned from the contralateral side placed over the femoral head can be used as a fluoroscopic guide. Using contrast injections, the common femoral artery is visualised and then punctured using a reference image. Alternatively, the tip of a catheter can be positioned in the targeted area guiding the puncture. Compared to these techniques, real-time angiography with DSA and road mapping allows the manipulation of the needle under fluoroscopy to obtain a “visually” guided puncture, resembling the surgical approach. It does not necessarily need the crossover step, which can sometimes be challenging.
Several predictors of vascular complications after transfemoral TAVI have been previously identified: early site and operator experience, moderate/severe iliofemoral artery calcification, minimal artery diameter, and a sheath to femoral artery ratio more than 1.056-8. The use of low-profile 14 to 18 Fr sheaths in the evolution of transfemoral TAVI has been associated with a drop in vascular complication rates19. The road mapping technique in this analysis was found to be associated with fewer major vascular and bleeding events. These results suggest that implementation of this simple technique, in addition to the ongoing development of smaller delivery systems, has the potential to decrease vascular and bleeding complications further and may impact on patients’ outcomes following transfemoral TAVI.
According to the current literature, both the Perclose ProGlide and the Prostar XL (Abbott Vascular) vascular closure devices are being used to close the femoral artery percutaneously after TAVI. It has been previously reported that the “double ProGlide technique” is associated with a low incidence of early and late closure site complications20. In this study, we used predominantly (98.75%) a single suture-based ProGlide closure device, which was successfully predeployed in all patients before the large sheath was introduced. There are scarce data about the use of this single device for the percutaneous vascular approach during TAVI. Kahlert et al reported, in a small number of patients, that preclosure of the arterial access site with this device was relatively easy, safe, and efficient: only one percutaneous closure device failure was reported (98.9% success)21. In our study, we achieved a 98.1% success rate in vascular closure with one device in the RM group (two patients requiring two devices and one device failure) and a 97.5% success rate in the control group (two patients requiring two devices and two device failures), which reinforces the safety and efficiency of this simple approach for access-site closure.
Another point worth mentioning regarding the benefits of road mapping is the possible reduction in contrast media utilisation during TAVI. In this analysis, a significant reduction in contrast use was observed in the road map group compared to the control group (200.2±55.1 vs. 251.9±84.1 ml, p<0.001 respectively). However, these results should be interpreted cautiously as the type of prosthesis and operator experience may have an impact. Self-expanding devices, which were more frequently used in the control group, usually require more contrast load during implantation compared to balloon-expandable valves.
Study limitations
The study has all the limitations of a single-centre registry and is therefore subject to possible bias and confounding. Though all parameters were prospectively collected in a dedicated institutional TAVI database, the analyses were performed retrospectively. The different types of sheath used due to the variability in implanted valves adds to the limitations of this study. Although exclusion of patients from the very early experience was an attempt to reduce the impact of the learning curve on measured outcomes, comparison of a more contemporary group to an older one might still be fraught with bias that may not be entirely correctable. Finally, due to the relatively small number of events observed, a multivariate analysis would have been overfitted and was therefore not performed.
Conclusions
A modified femoral artery puncture technique using DSA and road mapping was associated with a reduction in major vascular and bleeding complications after transfemoral TAVI, and provides a simple and effective strategy for potentially improving patient outcomes.
| Impact on daily practice The use of large sheaths in an elderly population has led to high rates of vascular and bleeding complications during transfemoral TAVI procedures. Therefore, a percutaneous accurate vascular access with true anterior wall entry in a disease-free segment of the common femoral artery is very important. Several methods to obtain a correct puncture site are applied in daily clinical practice. The visually guided modified femoral artery puncture technique using DSA and road mapping is a simple and effective strategy which can potentially have a positive impact on patients’ outcomes by reducing vascular and bleeding complications during transfemoral TAVI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

