
Abstract
Aims: Our aim was to assess the feasibility and results of a hybrid approach with a bioresorbable scaffold (BRS) plus a drug-coated balloon (DCB) for the treatment of diffuse coronary artery disease (CAD).
Methods and results: A retrospective analysis was performed on consecutive patients with diffuse de novo or in-stent restenosis treated with BRS implantation (larger proximal segment) and DCB inflation (smaller distal segment or bifurcation side branch). Endpoints were procedural success, then ischaemia-driven target lesion revascularisation (ID-TLR) and BRS/DCB segment thrombosis rates at follow-up. A total of 42 consecutive patients were treated with the hybrid strategy. Mean patient age was 62±1.02 years, while 12 (28.6%) patients were diabetics. Mean BRS and DCB length were 28.0±5.1 mm and 25.8±8.8 mm, respectively. Procedural success was obtained in all patients, but three (7.3%) patients required bail-out scaffolding for DCB-related dissection. At a median follow-up of 12 months (IQR: 6-18), there were no cases of cardiac death, target vessel myocardial infarction, or BRS/DCB segment thrombosis. ID-TLR occurred in two (4.7%) BRS-treated segments.
Conclusions: Our data in consecutive patients with diffuse CAD suggest that a hybrid strategy using BRS and DCB in different segments of the diseased vessel is feasible and associated with encouraging clinical outcomes.
Introduction
The everolimus-eluting bioresorbable vascular scaffold (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) is an intracoronary prosthesis which provides drug delivery and temporary scaffolding, and, over a period of three years, is fully reabsorbed by biochemical reactions, thus restoring the native vessel state1,2. The ABSORB trials have shown the reliable performance of this device in patients with stable angina or silent ischaemia due to relatively simple lesions, with many physiological advantages over permanent metallic prostheses and a low rate of major adverse cardiovascular events up to four years of follow-up3.
Currently, bioresorbable scaffold (BRS) use is expanding from simple to more complex lesions, and encouraging clinical results have been reported despite the technical challenges encountered3-5. On the other hand, drug-coated balloons (DCB) similar to BRS provide drug delivery without leaving a permanent structure at the lesion site and have been associated with favourable outcomes after treatment of bare metal stent (BMS) in-stent restenosis (ISR) or small vessel disease6,7. Of note, the common advantage of these two devices when used together (“hybrid strategy”) is the fact that no metallic cages are permanently left behind after their use. This fascinating aspect could be potentially effective in patients with diffusely diseased vessels involving small segments (distally or at a side branch of a bifurcation) unsuitable for bypass graft anastomosis. Furthermore, this strategy could be associated with long-term benefits for the vessel such as restoration of endothelial function, vascular healing and positive remodelling. Therefore, the objective of this study was to assess the feasibility and the clinical performance following the treatment of complex de novo or restenotic diffuse coronary artery disease involving small distal segments or side branches of a bifurcation using a hybrid strategy that overlaps or slightly superimposes BRS implantation and DCB inflation in different segments of the diseased vessel.
Methods
A retrospective, multicentre (six hospitals) cohort analysis was performed on all consecutive patients undergoing PCI with a “hybrid strategy” for stable or unstable coronary artery disease (CAD) due to complex lesions located in the same vessel. A hybrid strategy was defined as overlapping or slightly (2-3 mm) superimposing BRS (ABSORB BVS 1.1) implantation for a de novo or in-stent restenosis (ISR) lesion (located in the larger, more proximal part of the vessel) and DCB inflation for a concomitant de novo or ISR small vessel disease (located in the smaller distal segment or at a side branch of a bifurcation in the same coronary artery).
Complex coronary lesion was defined as the presence of de novo or restenotic diffuse (>25 mm in length) disease involving small distal segments or bifurcation side branches (SB) with a reference vessel diameter (RVD) more than 2.0 mm and less than or equal to 2.75 mm.
The rationale of the proposed strategy derives from the common characteristics of both DCB and BRS in treating the atherosclerotic disease without leaving a permanent structure in the vessel wall. This aspect could be important in case of diffuse disease in order to avoid a permanent cage within long coronary segments.
In particular, a DCB was considered suitable for the treatment of small segments (based on the BELLO trial results7), while BRS was used for the transient scaffolding of larger segments. This strategy avoids the risk of overlapping the thick BVS struts and reducing the minimum lumen diameter of small vessels by 600 µm.
The decision to perform a “hybrid strategy” rather than “conventional” permanent metallic stent implantation was left to the operator’s discretion in the presence of the aforementioned lesion characteristics.
All patients undergoing PCI for stable CAD were pre-treated with aspirin (100 mg/d). All patients undergoing emergent PCI for unstable CAD received a loading dose of antiplatelet drugs (i.e., aspirin 250-500 mg i.v. and either oral clopidogrel 600 mg or ticagrelor 180 mg or prasugrel 60 mg) periprocedurally. The dual antiplatelet therapy (DAT) regimen at discharge consisted of aspirin (100 mg daily) recommended indefinitely, in association with clopidogrel or ticagrelor or prasugrel for at least six months.
All patients were carefully informed about the alternative treatment options and the PCI-related risks before being asked to give written informed consent to the procedure. This was an observational and retrospective study, performed according to the privacy policy of the various institutions which participated and to their regulations for the appropriate use of data in patient-oriented research, which are based on international regulations, including the Declaration of Helsinki.
The primary endpoint of the study was procedural success, defined as a residual stenosis less than 30% at the BRS or DCB-treated segment without in-hospital major adverse cardiovascular events (a composite of cardiac death, target vessel Q-wave myocardial infarction [TV-MI] or need for emergent target lesion revascularisation [TLR]). Furthermore, we evaluated the occurrence of ischaemia-driven TLR (ID-TLR) and BRS/DCB-treated segment thrombosis at follow-up. Clinical events were defined according to the Academic Research Consortium definitions8. Clinical data were collected by hospital visit or telephone contact. Angiograms were analysed by means of the Clinical Measurements Solutions system (QCA-CMS, version 5.1; Medis medical imaging systems bv, Leiden, The Netherlands). Angiographic follow-up was scheduled or performed in case of staged revascularisation or if clinically indicated according to the policy of each institution. The investigators of each centre who participated in the study were asked to complete a structured patient-level database, including a series of clinical and procedural data as well as the clinical outcome data. Such individual patient data were anonymised and sent to the study coordinator (A. Ielasi), who was responsible for checking data consistency and for final pooling in a single database. Source verification and queries generation from the coordinating centre to the participating sites were undertaken to account partly for the unavoidable bias of site-reported event adjudication.
Statistical analysis
The values are presented as mean±standard deviation or median (interquartile range [IQR]) for continuous variables or as counts and percentages for categorical variables. All analyses were conducted using SPSS software, Version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Between May 2012 and December 2014, 42 consecutive patients underwent PCI with a “hybrid strategy” at the participating centres. Among these patients, the majority (n=37, 88.1%) had de novo diffuse disease (of whom two had chronic total occlusions) (Figure 1), while five (11.9%) had a diffuse BMS ISR (Figure 2).
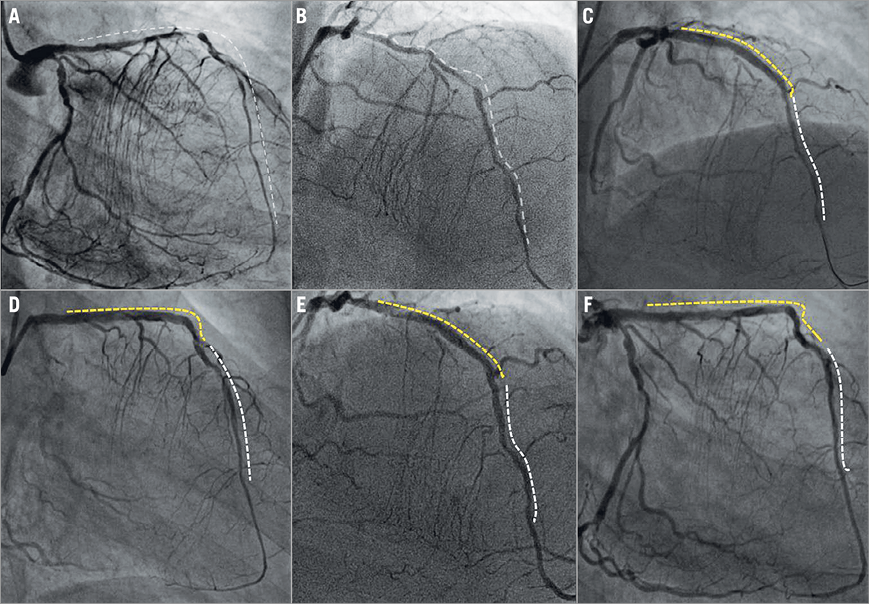
Figure 1. Hybrid strategy for the treatment of very long and diffuse LAD disease. Baseline right oblique and right cranial views showing diffuse left anterior descending (LAD) artery disease from distal to proximal segment (A & B). Final results following implantation of three overlapping 3.5×18 mm bioresorbable scaffolds (yellow dotted line) from mid to proximal LAD and two (2.5×30 mm) drug-coated balloons (white dotted line) inflated in the distal LAD (C & D). Twelve-month angiographic follow-up showing persistent patency of the LAD segments treated with the hybrid strategy (E & F).
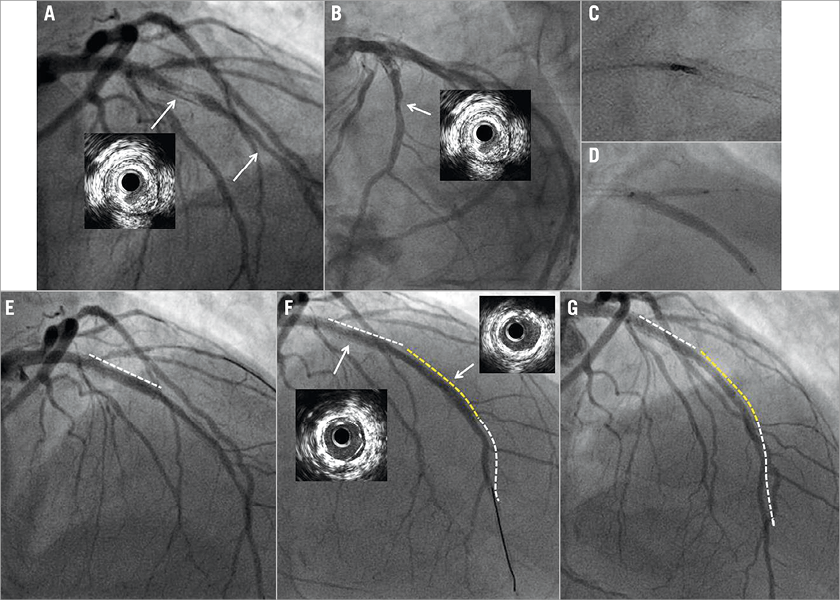
Figure 2. Hybrid strategy for the treatment of in-stent restenosis associated with distal small vessel disease. Baseline cranial views showing bare metal stent restenosis associated with mid and distal de novo left anterior descending (LAD) artery disease (A & B). In-stent directional atherectomy of the neointimal hyperplasia followed by 3.0×30 mm drug-coated balloon (DCB) dilatation and kissing balloon inflation at the LAD bifurcation with the diagonal branch (C & D). Final results following in-stent DCB (white dotted line) inflation (E) plus 2.5×28 mm bioresorbable scaffold implantation (yellow dotted line) and 2.25×30 mm DCB inflation at the mid and distal LAD (white dotted line) segments (F). Seven-month angiographic follow-up showing patency of the LAD segments treated with the hybrid strategy (G).
In two procedures, a BRS was implanted at the mid-proximal segment of the vessel as a bail-out strategy in the setting of an attempt of “DCB alone” treatment while, in all the other procedures, BRS (mid or proximal segment) and DCB (distal segment or SB of a bifurcation) were used as an intention-to-treat approach.
Details regarding baseline clinical characteristics of the patients evaluated are summarised in Table 1, while details regarding lesion and procedural characteristics are summarised in Table 2. Regarding DCB type, the IN.PACT Falcon™ (Medtronic, Minneapolis, MN, USA) was used in 23 (54.7%) patients, followed by the Pantera Lux (Biotronik, Berlin, Germany) in 12 (28.5%) and the SeQuent® Please (B. Braun, Melsungen, Germany) in seven (16.6%) patients.
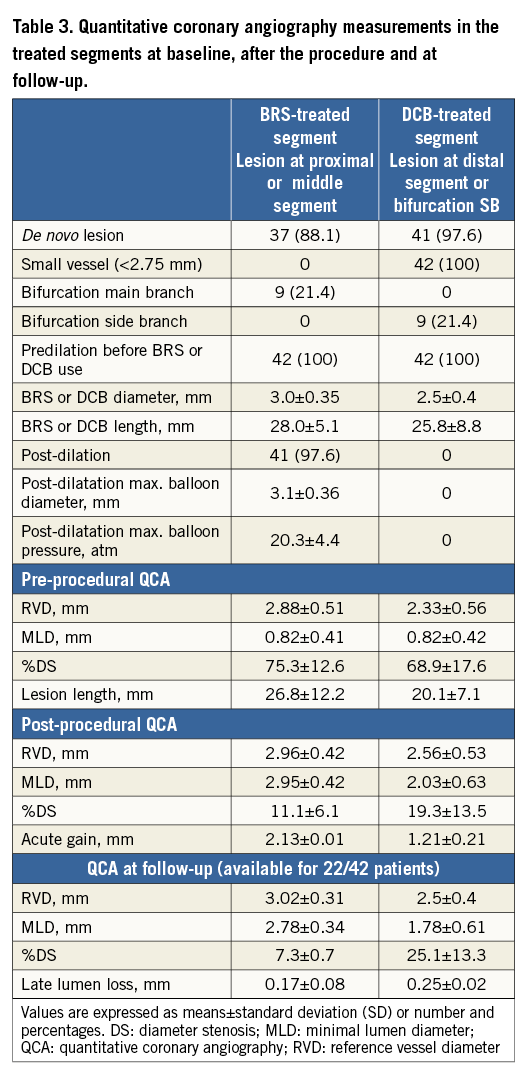
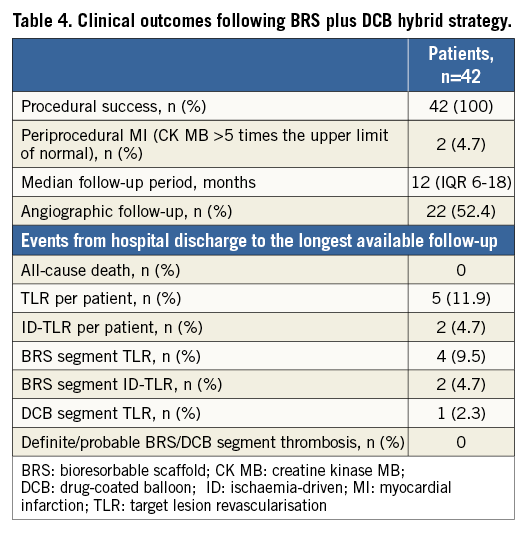
Quantitative coronary angiography measurements in the treated segments are reported in Table 3. Dual antiplatelet therapy was prescribed for 12 months after the procedure in 33 (78.6%) patients and for six months in the others. Aspirin and ticagrelor was the most frequent association (n=18, 42.9%), followed by aspirin and clopidogrel (n=16, 38.1%) and aspirin and prasugrel (n=8, 19%). Clinical outcomes following the “hybrid” strategy are reported in Table 4. Procedural success was obtained in all cases, but three (7.3%) patients required bail-out stenting (all these cases were managed with BRS implantation) for DCB-related extensive dissection. Periprocedural non-Q-wave MI (CK-MB more than five times the upper limit of normal) occurred in two (4.7%) patients. All the patients had at least one follow-up contact. At a median follow-up time period of 12 (IQR 6-18) months, no cardiac death and/or TV-MI was reported. Angiographic follow-up was performed in 22 (52.4%) patients. The angiographic control was clinically driven in two (9.1%) cases, while the others (n=20, 90.9%) were scheduled at discharge. TLR occurred in five (11.9%) patients and ID-TLR in two (4.7%) patients. BRS-treated segment TLR occurred in four (9.5%) patients, and BRS-treated segment ID-TLR in two (4.7%) patients. DCB-treated segment TLR occurred in one (2.3%) patient. All the BRS/DCB “failures” were successfully treated with re-PCI. No episode of definite/probable BRS or DCB-treated segment thrombosis was reported in-hospital or at follow-up.
Discussion
The main finding of this study is that a “hybrid” strategy with BRS implantation (at the proximal or middle segment of a coronary artery) and DCB inflation (at the distal segment or at a side branch of a bifurcation lesion in the same coronary artery) could be an acceptable approach for the treatment of de novo or restenotic diffuse CAD in the same vessel. In particular we showed:
1. The feasibility of a “hybrid” approach with a procedural success of 100%.
2. A relatively low occurrence of BRS/DCB-treated segment ID-TLR (4.7%) without segment thrombosis at a median of 12 months of follow-up.
The length of a metallic stent is a variable well known to be associated with a high risk of ISR and stent thrombosis (ST) at early and long-term follow-up9,10. In addition, the implantation of long permanent metallic prostheses in coronary vessels may impair restoration of vasomotion in the treated segment, promote neoatherosclerosis, and limit access for coronary artery bypass graft (CABG). A large single-centre experience previously demonstrated that treatment with more than 60 mm overlapping permanent DES (“full metal jacket” PCI procedure), although associated with acceptable mortality and ST rates, can lead to high TLR rates, approximating 24%11. For this reason, an interventional strategy that reduces the overall stent length could theoretically be of importance in the setting of diffuse, de novo or restenotic disease involving small distal vessels or side branches of a bifurcation, particularly when taking into account the high rate of restenosis after stenting in these settings10. The possibility of using DCB as part of a hybrid procedure combined with DES to limit stent length in vessels with diffuse disease was recently evaluated as an alternative approach12. Results following the treatment of 34 long lesions (>25 mm) using both permanent DES (in the larger, more proximal lesion site) and DCB (in the more distal, smaller part) showed the efficacy of this strategy and was associated with an 8.8% incidence of TLR (two cases in the DES-treated segment and one case in the DCB-treated segment) at a median of 26 months of follow-up. Of interest, no thromboses were reported in the treated segments in this cohort of patients (46.4% diabetics)12. As an evolution of the DES/DCB hybrid strategy, the first experience combining the use of two “leaving nothing behind” devices such as BRS and DCB in patients with diffuse de novo or restenotic disease in the same coronary vessel was reported last year in eight patients13 and has now expanded in the present study in 42 patients.
Despite some current limitations (i.e., the strut thickness for BRS and the relatively lower trackability of DCB compared to the current DES), both BRS and DCB showed very interesting clinical results at midterm and long-term follow-up after the treatment of de novo and restenotic disease14,15. Despite the relatively small cohort evaluated, the high procedural success rate reported in our study showed the technical feasibility of this strategy and it was not associated with particular complications. However, it is important to highlight that bail-out stenting could be required in the DCB-treated segments because of the presence of extensive dissection after prolonged inflation. In our study, the incidence of bail-out stenting was 7.3%, which was comparable to the 7.4% reported in a population treated with the DES/DCB strategy12. At a median of 12 months of clinical follow-up, ID-TLR per patient was 4.7% while TLR per patient was 11.9%. These rates, particularly the ID-TLR, suggest a good performance of the BRS/DCB strategy as compared to a DES/DCB strategy, even if a higher percentage of diabetics (28.6% vs. 46.4%) with longer lesions (37.9±9.1 mm vs. 67.7±13.4 mm) was treated with the latter approach. On the other hand, our TLR rate may appear higher compared to that reported following the treatment of patients with diffuse disease using a “DES alone” strategy in the LONG DES III (cobalt-chromium everolimus-eluting stent: 3.1% vs. sirolimus-eluting stent: 2.2%), IV (zotarolimus-eluting stent: 1.6% vs. sirolimus-eluting stent: 2.4%) and V (biolimus-eluting stent: 3.3% vs. platinum chromium everolimus-eluting stent: 2.0%) trials. However, it is important to note that, in the LONG DES trials, larger vessels (mean reference vessel diameter 3.2±0.4 mm) with only de novo disease were treated despite the similar prevalence of diabetes mellitus (about 30% of the population)16-18. Furthermore, it is important to underline that all the BRS/DCB “failures” reported in our cohort were successfully managed with re-PCI. Even if the number of patients treated was limited, making every consideration on the safety profile of the BRS/DCB approach preliminary, no thromboses were reported in the treated segment as compared to the DES/DCB “hybrid” approach, while the ST rate ranged between 0.4% and 0.8% in the aforementioned trials where a permanent “DES alone” strategy was adopted for the treatment of diffuse disease. Based on these results, the hybrid BRS/DCB strategy could be considered as a potential alternative approach to DES for vessel reconstruction in diffuse coronary disease with involvement of small segments. This modern approach in such circumstances could reduce overall stent length and stent use in small vessels, both of which are predictors of ST and TLR, while treating the underlying disease and theoretically maintaining access for a future CABG if required.
Limitations
This multicentre experience has some limitations deriving from the relatively small and heterogeneous cohort of patients treated, the observational nature of the study and the lack of a direct comparison versus conventional strategies. In particular, with the small cohort of patients, it is difficult to comment on the definite safety of the strategy, although we had no episodes of definite or probable BRS/DCB-treated segment thrombosis. The relatively limited follow-up period, the absence of a systematic scheduled angiographic follow-up and the possible selection bias due to the fact that the treatment strategy for the diffuse disease was left to the operator’s discretion preclude reaching definitive conclusions in terms of clinical outcome. Nevertheless, this is the largest reported study of such a novel strategy and the initial results are encouraging.
Conclusions
In conclusion, our preliminary data in patients with complex de novo and restenotic diffuse disease suggest that a hybrid strategy using BRS implantation and DCB inflation in different segments is feasible. Further larger studies of comparison versus more conventional strategies are warranted for better assessment of this attractive treatment option.
| Impact on daily practice The length of a metallic stent is a well-known predictor of in-stent restenosis and stent thrombosis at follow-up. In addition, the implantation of a long permanent stent within the coronary wall may impair restoration of vasomotion, promote neoatherosclerosis, and limit access for coronary artery bypass grafts. On these grounds, an interventional “hybrid” strategy that overlaps or slightly superimposes BRS implantation and DCB inflation (“leaving nothing behind” devices) in different segments of a diffusely diseased vessel may be an alternative to reduce the overall stent length while treating the underlying atherosclerosis and theoretically maintaining access for a future surgical revascularisation if required. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

