
CASE SUMMARY
BACKGROUND: A 71-year-old woman had a scheduled optical coherence tomography (OCT) (ILUMIEN™; St. Jude Medical, St. Paul, MN, USA) follow-up two years after implantation of a 3.0×18 mm bioresorbable vascular scaffold (BVS) (Absorb™; Abbott Vascular, Santa Clara, CA, USA) in the proximal left anterior descending coronary artery. She had dyspnoea corresponding to New York Heart Association (NYHA) Class II.
INVESTIGATION: Diagnostic angiography, OCT and fractional flow reserve (FFR) measurement.
DIAGNOSIS: OCT showed a covered and embedded BVS (not yet fully resolved after 24 months) and a distal edge in-scaffold restenosis and diffuse disease with an FFR of 0.74.
MANAGEMENT: The BVS edge stenosis and the segment with diffuse disease distal to the previously implanted BVS were treated with an additional Absorb BVS 3.0×28 mm overlapping with the BVS implanted two years earlier. Supplementary OCT showed a large intra-scaffold dissection proximal to the overlapping BVS segment; the dissection flap contained the previously implanted BVS.
KEYWORDS: bioresorbable vascular scaffold, Absorb, optical coherence tomography, intra-scaffold dissection
PRESENTATION OF THE CASE
A 71-year-old woman had a scheduled optical coherence tomography (OCT) (ILUMIEN™; St. Jude Medical, St. Paul, MN, USA) follow-up two years after implantation of a 3.0×18 mm bioresorbable vascular scaffold (BVS) (Absorb™; Abbott Vascular, Santa Clara, CA, USA) in the proximal left anterior descending coronary artery (LAD) and treatment with dual antiplatelet therapy (aspirin and clopidogrel) for 12 months, followed by aspirin as monotherapy. She had dyspnoea corresponding to New York Heart Association (NYHA) Class II, which was analogous to the symptoms she claimed prior to the index percutaneous coronary intervention (PCI). The echocardiogram, a pulmonary function test and chest X-ray examinations were normal.
At the two-year follow-up after Absorb BVS implantation, the angiogram showed a borderline lesion distal to the previously implanted Absorb BVS, and a diffusely diseased LAD. Following administration of 200 µg of intracoronary nitroglycerine, a diagnostic OCT showed a generally well-expanded BVS, completely covered in its entire length. Apart from two (malapposed) struts located at an aneurysmatic segment mid-distally, the BVS was well apposed. The scaffold was not fully resorbed at 24 months1,2, and the bioabsorption process was mostly progressed at the proximal BVS segment (Figure 1). The distal diffusely diseased lesion was characterised by several calcific noduli (Figure 1A) and lipid-rich plaques. One and a half centimetres distal to the most distal BVS segment, there was a severe, concentric, fibro-fatty plaque causing a minimal lumen area (MLA) of 1.1 mm2 (Figure 1B). More proximally, a small rupture site in relation to an eccentric, lipid-calcific plaque and the departure of a small septal side branch were revealed (Figure 1D). At the distal BVS reference segment site, there were calcified plaques, and a thin-cap fibroatheroma (TCFA) with a cap thickness of 50 µm was also found. In relation to the TCFA, erosions were visualised (Figure 1E), and the MLA was estimated to be 2.5 mm2. Overall, the appearance of the neointimal hyperplasia (NIH) coverage of the BVS was homogeneous. However, at the proximal part of the BVS, an area with porous NIH was noted (Figure 1G).
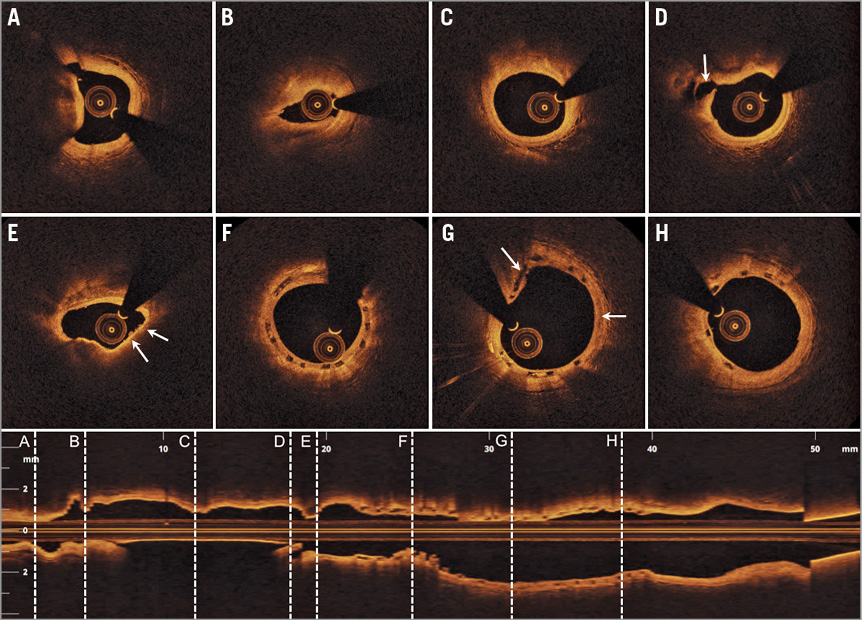
Figure 1. Diagnostic optical coherence tomography (OCT) of the Absorb BVS (3.0×18 mm) implanted 24 months earlier. Eight OCT cross-sections with locations (A-H) highlighted in the corresponding longitudinal OCT image. A) Calcific nodulus at the distal part of the diffusely diseased left anterior descending artery (LAD). B) A severe concentric fibro-fatty plaque causing considerable lumen compromise with a minimal lumen area (MLA) of 1.1 mm2. C) An eccentric fibrous plaque. D) Disruption of the luminal contour in relation to a fibro-calcific plaque and the departure of a septal side branch (arrow). E) Distal edge in-scaffold restenosis due to a calcified and lipid-rich plaque. Note, there is a thin-cap fibroatheroma (TCFA) (arrow) with associated erosions of the intimal coronary vessel wall surface (arrow). F) Distal BVS segment with covered and embedded struts. G) Mid BVS segment with covered and embedded struts. Note the bioabsorption from “2 o’clock to 4 o’clock” (arrow), and the porous appearance of the neointimal coverage at “10 o’clock to 11 o’clock” (arrow). H) Proximal BVS segment with homogeneous neointimal coverage and partial bioabsorption of the struts.
A fractional flow reserve (FFR) measurement during reactive hyperaemia with adenosine was 0.74. Based on combined OCT and FFR assessment, it was decided to treat the distal lesion with a new 3.0×28 mm BVS, overlapping with the previously implanted BVS. A loading dose of 600 mg of clopidogrel, and an unfractionated heparin dose of 6,000 units were administered. The edge in-scaffold restenosis and the distal segment with diffuse disease were predilated with a 2.5×20 mm semi-compliant balloon at 14 atmospheres (atm), and an angiographic result with less than a 40% diameter stenosis was achieved. The new 3.0×28 mm Absorb BVS was implanted with limited scaffold overlap, and 16 atm pressure was applied. The BVS-treated segment was further post-dilated with a 3.25×20 mm non-compliant balloon (16 atm). The angiogram showed haziness just proximal to the scaffold-overlapping segment, and a supplementary OCT was performed. The OCT revealed a large intra-scaffold dissection at the angiographically ambiguous “old” BVS segment (Figure 2). The “effective lumen area” (defined as the “true lumen” excluding the behind flap area) was measured at 4.70 mm2. The dissection disrupted both the neointima, the “old” BVS, and the native intima, and extended through to the native medial vessel wall layer. The circumferential extent was considerable, as the dissection arc was 140˚. Furthermore, flap length (measured from its tip to the joint point with the vessel wall) was 2.52 mm, flap root thickness (measured as a straight line from the joint point of the vessel wall to the luminal contour) was 0.71 mm, and flap area was 1.13 mm2. The longitudinal length of the intra-scaffold dissection was 1.6 mm. A limited dissection cavity extended 0.6 mm proximal to the intra-scaffold dissection site (Figure 2H). Besides these abnormalities, the OCT revealed an overall well-expanded and well-apposed BVS (Figure 2C-Figure 2F). At the distal reference segment site, a minor, medial edge dissection with limited circumferential extent (dissection arc 70˚) was visualised.
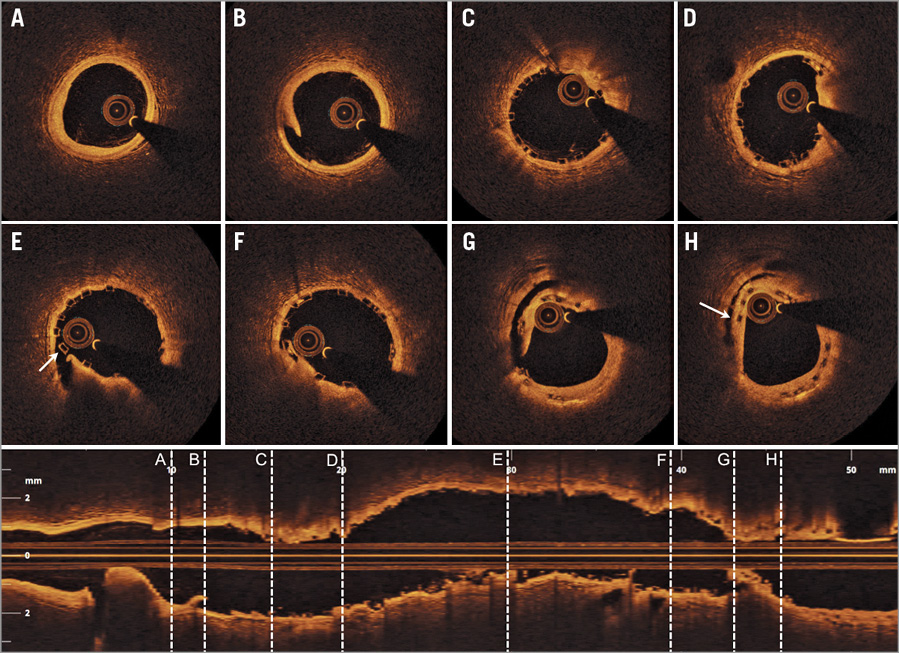
Figure 2. Supplementary optical coherence tomography (OCT) following implantation of a new BVS (3×28 mm) distally to the BVS implanted 24 months prior. Eight OCT cross-sections with locations (A-H) indicated in the corresponding longitudinal OCT image. A) Distal reference segment with slight intimal thickening, but otherwise inapparent coronary vessel wall. B) Minor, medial distal edge dissection at a normal coronary vessel wall site. C) Well-expanded distal BVS segment. D) Well-expanded distal-mid BVS segment. E) Mid BVS segment, where a strut at “9 o’clock” is malapposed due to underlying intra-scaffold dissection (arrow). F) Well-expanded mid-proximal part of BVS. G) A large intra-scaffold dissection with the dissection flap disrupting both the neointima, the “old” BVS, the native intima, and extending through to the native medial vessel wall layer. H) A short and limited intra-scaffold dissection cavity (arrow).
How would I treat?
THE INVITED EXPERTS’ OPINION

This interesting case describes the practical issues related to a stent edge dissection, which in this case occurred as an immediate complication of bioresorbable vascular scaffold (BVS) implantation. The scaffold edge dissection was immediately detected on angiography and confirmed by optical coherence tomography (OCT). Thanks to its exceptionally high spatial resolution, OCT detects a higher number of edge dissections as compared to angiography or intravascular ultrasound, but at the same time may generate more frequent concern for further associated complications and trigger additional stent implantation. Considering that OCT is increasingly used to guide coronary interventions, it is worth having a detailed discussion on this particular subject. The strain difference within the unevenly resolved old scaffold, augmented by the new BVS implantation process, may constitute a possible mechanism of the edge dissection in this case. However, the main factor will be the vessel wall overstretching during distal scaffolding, as evidenced by the considerable size discrepancy between the new distal BVS and the proximal old one identified by the longitudinal OCT image (Figure 2).
The dilemma on the subsequent management of the scaffold edge dissection in this situation originates from the morphometric characteristics of the dissection flap. Based on the previous IVUS or OCT studies3,4, the edge dissection in this case may not be “minor” (which usually has a benign course and is not associated with an increased incidence of adverse outcomes). The arc of dissection (140˚), flap length (2.52 mm) and flap root thickness (0.71 mm) are all considerable, and the vessel wall disruption extended through the medial layer. Although there is limited consensus regarding the morphometric OCT criteria to predict adverse events, these dramatic OCT findings may be evidence to perform additional mechanical treatment.
In our practice, we would pay attention to the positive aspects. The dissection directed proximally, in the opposite direction to the blood flow in the true lumen. This will prevent further propagation of the dissection, and may promote “gluing” the dissected layers together following the approximation of fluttering tissue flaps against the vessel wall. Also, the longitudinal extension of dissection is quite short, and the lumen area at the site of dissection is relatively maintained. Thus, as long as the flow is maintained (assessed by either angiogram or fractional flow reserve), we would prefer medical observation including dual antiplatelet therapy, and consider GP IIb/IIIa inhibitors to prevent early stent thrombosis. Of course, in case of a flow-limiting dissection, we preferably would use a metallic stent to seal up the dissection.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

The authors report a two-year bioresorbable vascular scaffold (BVS) distal-edge restenosis, which was treated with a second BVS implantation with minimal scaffold overlap. Although a second BVS was deployed following a meticulous implantation technique, some haziness was evident angiographically at the BVS overlapping segment. Optical coherence tomography (OCT) showed a large intra-scaffold dissection at the site of the “old” BVS segment, disrupting the neointima, the “old” BVS and the native intima with a longitudinal length of 1.6 mm.
In our opinion, the decision on how to treat it should be based on three important factors: the dissection extension, the presence of a proximal intimal tear, and the type of “old” stent included within the dissection.
Previous studies have shown that angiographically evident large dissections (types C to F) caused by stent implantation and left untreated are associated with unfavourable events at early and midterm follow-up, mainly due to high risk of stent thrombosis and major adverse cardiovascular events5. In the case herein presented, the dissection extension is considerable and angiographically visible.
The presence of a proximal tear is also important to decide about treatment. If left untreated, a proximal dissection flap could cause further dissection extension, just due to coronary flow, which conversely cannot extend the dissection further without a proximal tear6. In this case, a proximal tear inside the “old” BVS may be recognised.
Last, but not least, the type of previously implanted stent is also essential in taking the final decision on the best treatment. In this specific case, the dissection extends proximally, disrupting the neointima of a previously (two years before) implanted BVS. At this time point the BVS bioresorption is at an advanced stage: the polymer has been replaced by proteoglycan-rich matrix and the BVS has already lost its radial force7. We may hypothesise that a dissection involving the neointima of a metallic stent, which may still provide some radial force and mechanical support to the vessel wall, may be left as it is, always considering the absence of a proximal tear and the lack of lumen area compromise. On the other hand, in the present case, as the BVS scaffold does not exist anymore as a supportive structure, this dissection should be considered as a dissection in a native coronary artery with the risk that the disrupted neointima may collapse into the lumen, obstructing the flow.
Based on all these considerations, we would have treated this case by implanting a new stent in order to cover the “old” BVS, to eliminate the risk of neointima prolapse and flow compromise. In particular, we would have used a metallic DES, as no data are available on how BVS bioresorption is influenced by implantation on a vessel wall where another BVS is being bioresorbed. Moreover, delayed healing has been shown in case of BVS overlap, so that minimal or no overlap is recommended when two BVS are implanted side by side8.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The intra-scaffold dissection involving the segment with scaffold overlap needed to be covered. In covering a dissection involving scaffolds, the best management strategy remains to be elucidated and the concern was whether to implant a third Absorb BVS or a metallic drug-eluting stent (DES). Implanting an Absorb BVS would result in a triple layer of scaffolds. On the other hand, in-scaffold implantation of a metallic DES may result in the loss of the theoretical late advantage of a scaffold for the treated segment, including restored vasomotion and reduced risk of late thrombosis. It was decided to implant a new Absorb BVS (3.0×12 mm at 12 atm) covering the dissected part.
A final OCT was performed in order to visualise the sealing of the intra-scaffold dissection (Figure 3). Overall, the scaffold was acceptably expanded. There were a few malapposed struts at the previous intra-scaffold dissection site, but the malapposition size was of limited magnitude (maximal malapposition distance 350 µm). At the triple-layer BVS segment site (Figure 3A), the MLA was 5.28 mm² and, at the previously sealed intra-scaffold dissection site, the MLA was 5.48 mm² (Figure 3B).
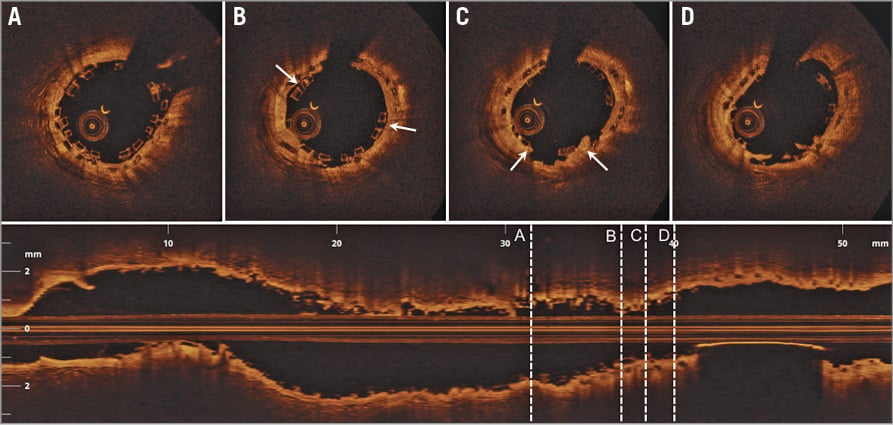
Figure 3. Final optical coherence tomography (OCT) following implantation of an additional BVS (3.0×12 mm) at the “old” intra-scaffold dissection site. Four OCT cross-sections with locations (A-D) indicated in the corresponding longitudinal OCT image. A) Triple-layer BVS. B) Previous intra-scaffold dissection site, where the flap has been sealed with an additional BVS. The BVS is slightly underexpanded, which results in slight malapposition at “3 o’clock and 10 o’clock” (arrows). C) Further proximal at the previous intra-BVS dissection location. Note the flap remnants from “4 o’clock to 7 o’clock” (arrows). D) Most proximal part of earlier intra-scaffold dissection site, where flap residuals are also visible at “5 o’clock to 7 o’clock”. Slight malapposition is present around “2 o’clock”.
Discussion
A triple layer of Absorb BVS has not previously been described in the literature. The Absorb BVS provides a temporary coronary scaffold and loses radial strength and structural continuity six months after implantation1, and it is thought to be resorbed by about two years9-13. Although remnants of scaffold boxes were visible on OCT images after two years, they no longer have the same function as metallic DES struts, which explains the risk of a scaffold edge dissection when treating a scaffold edge restenosis as in the present case.
Conflict of interest statement
L. Okkels Jensen has received research grants to her institution from St. Jude Medical and honoraria from Abbott Vascular and St. Jude Medical. The other authors have no conflicts of interest to declare.

