
Abstract
Aims: The aim of our study is to report our single-centre experience with concomitant MitraClip (MC) and left atrial appendage occlusion (LAAO) and further to assess the feasibility, safety and short-term outcome of such an approach.
Methods and results: Twenty-five consecutive patients underwent MC with concomitant LAAO at our hospital (combined group). As a control group, 25 consecutive patients with atrial fibrillation (AF) undergoing standalone MC were selected. Baseline parameters were equal between the two groups. Patients in the combined group had longer procedural time (90.0 min vs. 66.0 min, p=0.02) and radiation time (32.0 min vs. 18.0 min, p=0.01). There were no procedural deaths. At 30 days, one patient died due to cerebral haemorrhage (combined vs. control: 4% vs. 0%, p=0.32) and two had acute kidney injury (combined vs. control: 4% vs. 4%, p=1.00). In multivariate analysis, the association of LAAO with device or procedural success was not significant.
Conclusions: LAAO along with MC in a single stage procedure is feasible. These preliminary results have to be validated in a large randomised study, in order to assess the efficacy of combined LAAO that can be expected to become evident only after longer follow-up.
Abbreviations
AF: atrial fibrillation
LAAO: left atrial appendage occlusion
MC: MitraClip
MR: mitral regurgitation
PMVR: percutaneous mitral valve repair
Introduction
Percutaneous mitral valve repair (PMVR) with the MitraClip® (MC) system (Abbott Vascular, Santa Clara, CA, USA) has been established as the therapy of choice for patients with mitral regurgitation (MR) who are inoperable or high-risk surgical candidates1. Atrial fibrillation (AF) is a frequent comorbidity in patients undergoing PMVR, and many of these patients have contraindications for oral anticoagulation or are at high risk for major bleeding1,2. As compared to medical therapy, left atrial appendage occlusion (LAAO) results in lower rates of stroke (mainly by reducing the rate of haemorrhagic strokes) and resulted in improved survival after 3.8 years of follow-up3,4. Combining MC therapy and LAAO, which both require left atrial access, can be easily achieved from a technical standpoint. Therefore, combining these two therapies in one single procedure seems attractive, if it does not lead to an increase in complications. The aim of our study is to report our single-centre experience with combined MC and LAAO and to assess the feasibility, safety and short-term outcome of such an approach.
Methods
POPULATION
In this retrospective study, the outcomes of 25 consecutive patients who had undergone PMVR together with LAAO (combined group) at our hospital between March 2014 and June 2016 were assessed. Twenty-five consecutive patients with AF undergoing isolated MC therapy during the same period served as the control group (control group).
The indication for PMVR was discussed among the members of the Heart Team on the basis of estimated surgical risk and patient preferences. MR was graded according to the current guidelines of the European Society of Cardiology5. Demographic and clinical characteristics, including risk of stroke and major bleeding (CHA2DS2-VASc score, HAS-BLED score), procedural data and outcome data of in-hospital and follow-up periods were captured within the MitraSwiss registry6,7. The MitraSwiss registry was approved by the local ethics committee. All patients gave written informed consent. The decision to perform concomitant LAAO was based on a high bleeding risk and patient or physician preference.
PERCUTANEOUS MITRAL VALVE REPAIR
In all patients, PMVR was performed with the MC system. The procedure was performed under general anaesthesia and with transoesophageal echocardiographic (TEE) and fluoroscopic guidance.
LEFT ATRIAL APPENDAGE OCCLUSION
Left atrial appendage occlusion was performed after MC therapy using an AMPLATZER™ device (AMPLATZER™ Cardiac Plug [ACP] or Amulet™; St. Jude Medical, St. Paul, MN, USA). Using the same transseptal puncture site, a stiff wire was introduced to the left atrium and the MC system was removed. The 13 Fr (in the case of the ACP) or 14 Fr (in the case of the Amulet) 45°×45° AMPLATZER™ TorqVue™ delivery sheath (St. Jude Medical) was introduced and bleeding was controlled by either pulling on a ProGlide® (Abbott Vascular) in case venous preclosure was performed, or by a “figure of 8 stitch”. Using an RAO caudal view, the LAA was engaged with the AMPLATZER TorqVue delivery sheath. The diameter of the device landing zone was estimated on angiography, taking the outer diameter of the 13 Fr (5.1 mm) or 14 Fr (5.4 mm) sheath as a reference. Device deployment was carried out using fluoroscopic guidance. Stability of the device was assessed by a tug test and by angiographic imaging in views perpendicular to the device. Furthermore, TEE confirmed a stable and optimal device position. Oral anticoagulation was terminated on the day of the procedure. Acetylsalicylic acid was prescribed indefinitely and clopidogrel 75 mg was given for at least three months. A pre-discharge transthoracic echocardiogram confirmed a correct device position. TEE at three to six months excluded device thrombus and a residual leak.
OUTCOMES
Procedure times and amount of contrast were assessed for all patients. Procedural time was defined as the start time of the first venous puncture to the time of closure of the puncture site. The endpoints were defined according to the Mitral Valve Academic Research Consortium (MVARC) classification at 30 days8. Clinical follow-up data were obtained by review of medical records and telephone interviews.
STATISTICAL ANALYSIS
Data are expressed as median and interquartile range (IQR) for continuous variables and as a number and percentage for categorical variables. The Mann-Whitney U test was used to determine differences between the two groups for continuous variables, and the chi-square test was used to determine differences between the two groups for categorical variables. Logistic regressions were used to evaluate the independent prognostic value of LAAO categories on device and procedural success, after adjustment for demographics and conditions. A probability value of <0.05 was considered to indicate statistical significance. Analysis was performed using a standard statistical software programme (SPSS, Version 22; IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
Median patient age was 80 years (IQR 74.5-84.0 years) and the left ventricular ejection fraction (LVEF) was 54% (IQR 43-61%). Twenty-eight percent of patients had a history of myocardial infarction and 40% were known to have coronary artery disease. Thirty percent of patients suffered from degenerative MR. Baseline pre-procedural features of the two groups were comparable (Table 1). The aetiology of MR and number of clips implanted were also comparable between the two groups. Patients in the combined group had a longer procedural time (90.0 [IQR: 62.0-117.5] min vs. 66.0 [IQR: 54.5-89.5] min, p=0.02) and radiation time (32.0 [IQR: 23.0-41.0] min vs. 18.0 [IQR: 15.0-27.0] min, p=0.01) compared to the control group. LAAO was successful in all patients. Technical success was 100% in the overall study population (Figure 1).
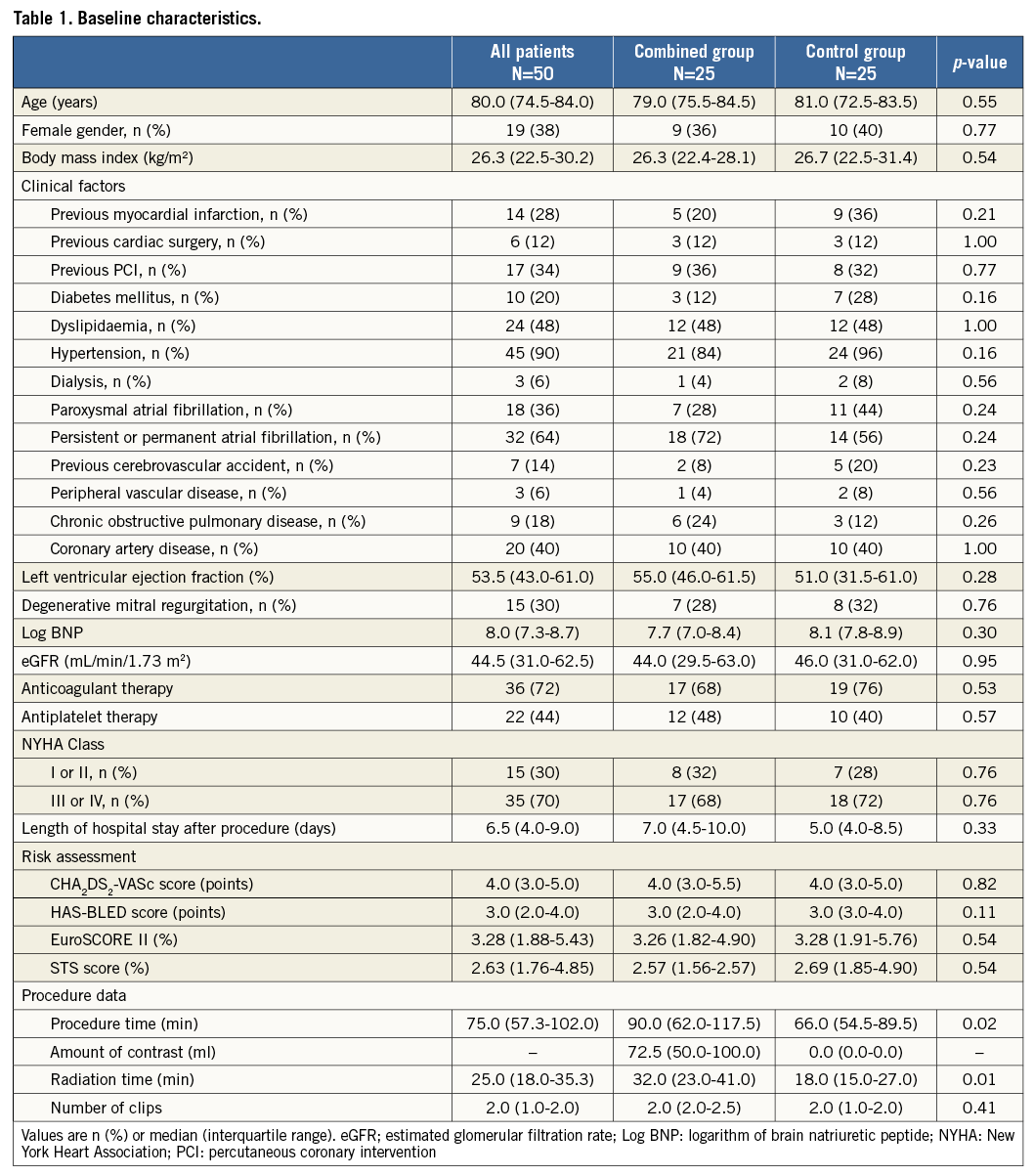
Figure 1. Postoperative results between the combined group and the control group. Red lines are percentages of postoperative results in the combined group and grey lines are percentages in the control group. There is no significant difference between both groups for all of them. MR: mitral regurgitation
CLINICAL FOLLOW-UP
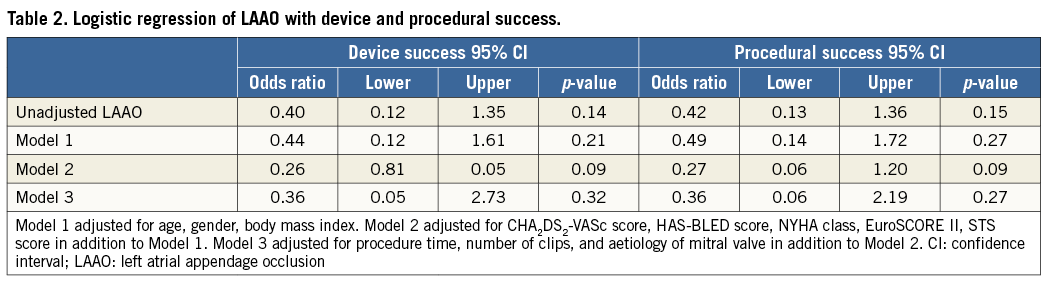
Length of hospital stay did not differ between the two groups (combined vs. control: 7.0 [IQR: 4.5-10.0] days vs. 5.0 [IQR: 4.0-8.5] days, p=0.33). At 30 days, one patient in the combined group died due to cerebral haemorrhage (combined vs. control: 4% vs. 0%, p=0.32). Three patients had a minor vascular access-site complication (combined vs. control: 4% vs. 8%, p=1.00), and two patients suffered acute kidney injury (combined vs. control: 4% vs. 4%, p=1.00). There was no significant difference between the combined group and the control group in other post-procedural events (Figure 1). There was no association of LAAO with device or procedural success (Table 2), both in univariate analysis (odds ratio [OR] 0.40, p=0.14; OR 0.42, p=0.15), or after multiple logistic regression analysis (OR 0.36, p=0.32; OR 0.36, p=0.27).
Discussion
In patients with degenerative MR undergoing mitral valve surgery, AF negatively affects late stroke rate and long-term survival9. On the other hand, in the EVEREST II trial, acute procedural success, procedural safety, and one-year efficacy with MC therapy were similar for patients with and without AF10. In this previous study, AF patients comprised fewer than 30% of all patients. Real-world patients undergoing MC therapy are often older and sicker than in the EVEREST II trial11: for example, in the ACCESS-EU study, AF was present in more than 65% of patients2.
It therefore comes as no surprise that survival after MC was worse in patients with AF and severe left ventricular dysfunction (LVEF ≤30%), as compared to patients in sinus rhythm12. Furthermore, we know from patients undergoing transcatheter aortic valve replacement that concomitant AF is associated with late cardiovascular morbidity and mortality13-15. Bleeding events may account at least in part for the worse outcome of AF patients16. Preventing bleeding complications is therefore an important matter17.
Combining LAAO with MC reduces the bleeding risk and therefore makes sense from a theoretical standpoint. So far, only case reports of concomitant MC and LAAO have been reported18,19. In our study, we demonstrated the feasibility and safety of such a combined approach. Despite longer procedural time compared to the control group, the combined group did not experience more adverse events. Alternatively, the two procedures could be staged in two single procedures. While staging the two procedures is better reimbursed in most countries, combined procedures seem more patient-friendly and possibly even safer than staged procedures (one single vascular access puncture, one single transseptal puncture, immediate cessation of oral anticoagulation).
Patients with chronic kidney disease (CKD) are well known to be at higher risk for procedural complications or worse clinical outcomes after transcatheter interventions20-22. Although the combined procedure requires a higher volume of contrast medium, the present study demonstrated that combining LAAO with MC did not have a significant impact on the incidence of acute kidney injury. A previous study on isolated LAAO using a mean contrast volume of 122 ml showed similar procedural safety among CKD patients compared to patients with normal renal function23. We could further show that there was no significant association when adding LAAO with respect to device or procedural success rates. From a theoretical standpoint, one could argue that the combination of the two procedures may result in a lower success rate of LAAO, given the less-than-ideal transseptal puncture site in some patients. According to our data and our own large experience with LAAO, good exclusion of the LAA can be achieved, even with a high transseptal puncture.
In our study, no ischaemic stroke, no pericardial effusion or cardiac tamponade occurred in the combined group. A haemorrhagic stroke occurred in one patient in the combined group. This underlines the fact that patients undergoing MC procedures, comprise a high-risk population for bleeding complications. Currently, the optimal peri-interventional anticoagulation regimen after MC remains unknown, underlining the lack of evidence in the field24-26. Patients undergoing MitraClip procedures are typically elderly patients with several comorbidities, hence they are at high risk for bleeding. This is particularly true if additional coronary artery disease with an indication for antiplatelet therapy is present, if patients suffer from chronic kidney disease, or if patients are multimedicated.
This study demonstrates the feasibility of combining LAAO with PMVR. Combining the two procedures seems particularly appealing, since both require left atrial access. This also applies to transcatheter mitral valve replacement therapies, where combination with LAAO seems equally attractive – if the access is transvenous transfemoral. Besides, combining LAAO with transcatheter aortic valve implantation has recently been shown to be safe27.
Limitations
Our study has some limitations. This is a retrospective single-centre study and not a randomised trial, and therefore a selection bias is evident. The sample size was small, which limits comparability. Furthermore, follow-up was short, not allowing any conclusions regarding the efficacy of combining the two approaches (e.g., better long-term survival). Longer-term follow-up and more patients are required to confirm these initial results in a randomised, large prospective study.
Conclusion
In patients with AF undergoing MitraClip implantation, concomitant left atrial appendage occlusion (LAAO) is feasible and safe. Success rates and 30-day outcomes did not differ between the combined (LAAO and MitraClip in the same procedure) and the control group (MitraClip alone).
| Impact on daily practice Patients undergoing percutaneous mitral repair with the MitraClip often suffer from AF and are at high risk for bleeding. In patients with AF undergoing MitraClip implantation, concomitant left atrial appendage occlusion is feasible and safe. Longer-term follow-up and more patients are required to confirm these initial results and will define a randomised, large prospective study. |
Conflict of interest statement
M. Taramasso is a consultant for St. Jude Medical. F. Maisano is a consultant for St. Jude Medical, Edwards Lifesciences, Abbott Vascular, Medtronic and Valtech, is the co-founder and a shareholder of 4Tech Cardio and receives royalties from Edwards Lifesciences. F. Nietlispach is a consultant for St. Jude Medical, Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

