
Abstract
Aims: The aim of this study was to evaluate the role of direct catheter-based thrombectomy (d-CBT, without thrombolysis) and the feasibility and safety of d-CBT performed in an interventional cardiology centre.
Methods and results: This single-centre, prospective observational registry based on the pre-specified protocol included three months of follow-up. The decision to perform acute stroke intervention was made by a neurologist based on the clinical and imaging findings. Inclusion criteria were moderate-to-severe acute ischaemic stroke (NIHSS ≥6), <6 hours from symptom onset, no large ischaemia on the admission CT scan and CT evidence for an occluded large artery. The primary outcome was functional neurologic recovery (mRS 0-2) at three months. Key secondary outcomes were the angiographic recanalisation rate and symptomatic intracranial bleeding. A total of 115 consecutive patients (mean age 66 years) were enrolled during a period of four years: 84 patients underwent d-CBT and 31 patients bridging thrombolysis with immediate catheter intervention (TL-CBT). The annual number of procedures increased from 13 (initial 12 months) to 41 (last 12 months). Angiographic success (TICI flow 2b-3) was 69% after d-CBT and 81% after TL-CBT. It was higher in isolated occlusions of the middle cerebral artery (MCA, 74% and 100%) or of the proximal internal carotid artery (ICA, 80% and 100%), while it was lower in combined ICA+MCA occlusions (63% and 70%) and in basilar or vertebral occlusions (57% and 50%). Neurologic recovery (mRS ≤2 after 90 days) was achieved in 40% of patients. It was higher (43%) in anterior circulation strokes than in posterior circulation strokes (25%). Direct CBT led to neurologic recovery in 36%, while in TL-CBT this was 52%. Best clinical outcomes (51% and 71% neurologic recovery rates) were achieved among patients with isolated MCA occlusion. Any symptomatic intracranial bleeding was present in 3.6% (d-CBT) and 6.5% (TL-CBT). Vessel perforation or major dissection occurred in 5.2% overall, and distal embolisation to other territory in 3.5% of patients.
Conclusions: Direct catheter-based thrombectomy may be considered in patients with contraindications for thrombolysis or in patients with very short CT-groin puncture times. A randomised trial is needed to evaluate better the role of direct catheter-based thrombectomy. Acute stroke interventions performed in close cooperation among cardiologists, neurologists and radiologists are feasible and safe.
Abbreviations
CT: computed tomography
d-CBT: direct catheter-based thrombectomy (without thrombolysis)
ICA: internal carotid artery
MCA: middle cerebral artery
mRS: modified Rankin scale
NIHSS: National Institutes of Health Stroke Scale
TICI: Thrombolysis In Cerebral Infarction
TL-CBT: bridging thrombolysis with immediate catheter intervention
Introduction
Direct (or primary, i.e., without thrombolysis) percutaneous coronary intervention (PCI) became an established nationwide treatment of acute myocardial infarction with ST-segment elevation (STEMI) in 2002 in the Czech Republic1,2 and in subsequent years also worldwide3-7. This significantly improved the outcomes of patients with STEMI.
Direct catheter-based thrombectomy (d-CBT) in acute ischaemic stroke is still in its infancy. Endovascular interventions for acute stroke developed rapidly after the introduction of the newer technology, i.e., stent retrievers8. Since the introduction of stent retrievers, five large randomised trials have collected clear evidence that endovascular interventions used on top of intravenous (bridging) thrombolysis significantly improve outcomes of patients with acute ischaemic stroke9-13. Thus, new guidelines for acute ischaemic stroke recommend bridging thrombolysis followed immediately by CBT as a class IA indication for patients with moderate-to-severe ischaemic stroke, who fulfil imaging and clinical criteria. Nevertheless, it remains unclear whether bridging thrombolysis adds to the benefit of CBT alone or vice versa. No randomised data exist to compare direct CBT with CBT performed after bridging thrombolysis.
An even greater question is whether (due to the limited availability of experienced neuroradiologists) acute stroke interventions could be performed at experienced interventional cardiology centres. Such a strategy may facilitate the availability of this modern treatment to a broad population, but it is not known at what price – whether the results of such cardiology centres could be comparable with experienced neuroradiology centres or whether such an approach may cause higher complication rates and lower success rates.
The aim of this study was to search for answers to both these questions: the role of direct CBT and the feasibility and safety of CBT performed in an experienced high-volume interventional cardiology centre.
Methods
SETTING
Our large tertiary university hospital had no neurointerventional programme until 2012. In 2010, the decision was taken to build such a programme with the close cooperation of cardiologists, neurologists and radiologists – the true interdisciplinary approach. Preparations included staff changes (new chair of neurology, interventional neuroradiologist joining full-time interventional cardiology staff), preparation of local protocols, one-month visit of the three key staff members to the high-volume comprehensive stroke centre in Buffalo (USA). Furthermore, because new approaches have been used (neurointerventions at an interventional cardiology department, direct CBT without thrombolysis), the study protocol was discussed and approved by the local ethics committee as with any other research protocol. The way interdisciplinary cooperation was organised and the route of patients through the hospital are demonstrated in Figure 1.
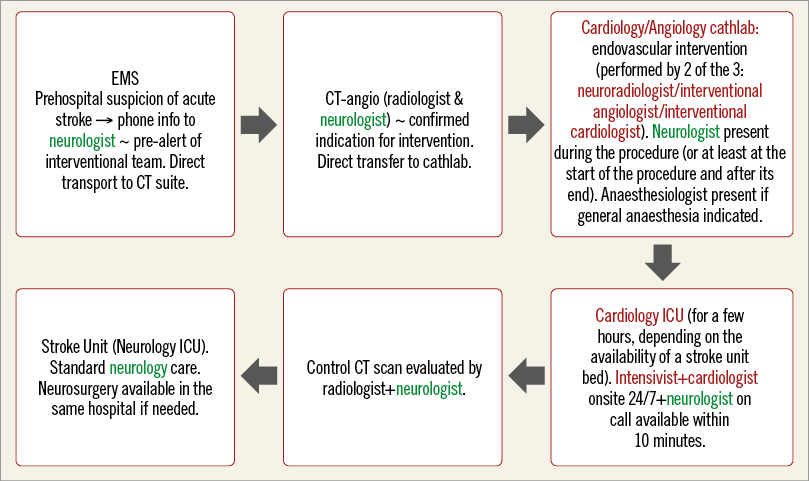
Figure 1. Patient flow through the hospital demonstrating interdisciplinary cooperation. The neurologist (in green) is responsible for all steps from indication through to post-interventional patient evaluation to rehabilitation. The Cardiology Department and staff (in red) serve only to perform the intervention and to take care of the patient for the few hours after the intervention – but even at this stage the neurologist is evaluating the patient.
STUDY DESIGN
This is a single-centre, prospective observational registry based on the pre-specified protocol and including a minimal follow-up of three months. This paper summarises the data after the first four years. The decision to perform acute stroke intervention was made by a board-certified neurologist in all patients based on the clinical and imaging findings.
Inclusion criteria were: moderate to severe acute ischaemic stroke (NIHSS ≥6), time interval <6 hours from symptom onset, no or only small ischaemia visible on the admission CT scan, CT evidence for an occluded major artery (either CT-angio or dense artery sign on CT scan) and expected ability to start intervention within <60 minutes from CT. Exclusion criteria were: previously known neurologic symptoms (mRS 2-5), known coagulation disorders, known severe hypoglycaemia, intracranial bleeding, CT evidence of large ischaemia.
The primary endpoint was functional neurologic outcome (mRS) at three months (assessed by board-certified neurologists). Secondary outcomes were: angiographic recanalisation rate, change of the National Institutes of Health Stroke Scale score (ΔNIHSS) from admission to discharge, symptomatic intracranial bleeding (defined as ΔNIHSS ≥4 within 48 hours after intervention).
Endovascular interventions were performed via the femoral approach in all patients. The carotid artery was engaged first with a diagnostic catheter, and digital subtraction angiography of the diseased side was performed. Once intracranial occlusion or critical flow-limiting stenosis had been confirmed, the carotid artery (in anterior strokes) was cannulated with a balloon guide catheter. A 0.010” guidewire (or 0.014” in some patients) was used together with a microcatheter to cross the occlusive thrombus. Then the guidewire was replaced by a stent retriever which was deployed across the thrombus, left in place for three to five minutes and withdrawn while the carotid artery was temporarily occluded by the balloon guide, and 50 cc syringe aspiration was carried out simultaneously with clot retrieval. If necessary, the procedure was repeated. In patients with proximal (extracranial) internal carotid occlusion (or flow-limiting stenosis), carotid stenting was performed (Figure 1). In five patients, large bore aspiration catheters were used: in two before, in one after and in two without stent retrievers. Most interventions were performed by two operators out of the three involved in this study – one interventional neuroradiologist (B. Koznar), one interventional angiologist (F. Rohac) and one interventional cardiologist (P. Widimsky), all three being members of the cardiology staff. This mode of operation was selected in order to facilitate the staff experience facing the limited procedure volume per year – especially during the first two years.
ANTITHROMBOTIC TREATMENT
The use of bridging thrombolysis (tPA i.v. in guideline-recommended dosage) was left to the discretion of the attending neurologist. As the study protocol recommended direct CBT, thrombolysis was usually used in situations when the immediate start of the endovascular intervention was less certain (e.g., during nights or weekends, when the interventionalist had to travel from home to the hospital). Heparin was only rarely used in thrombolysed patients and, if used, the dose was just 20-25 U/kg. In d-CBT patients, a low dose of heparin (25-40 U/kg) was used routinely during the procedure. All patients received aspirin the next day after the procedure unless a bleeding complication was detected on CT. Clopidogrel was added on top of aspirin only in patients with acute phase carotid stenting.
NEUROLOGIC FOLLOW-UP
All patients were followed by a neurologist prior to, during and after the procedure. They were also invited for neurologic and cardiologic control after three months.
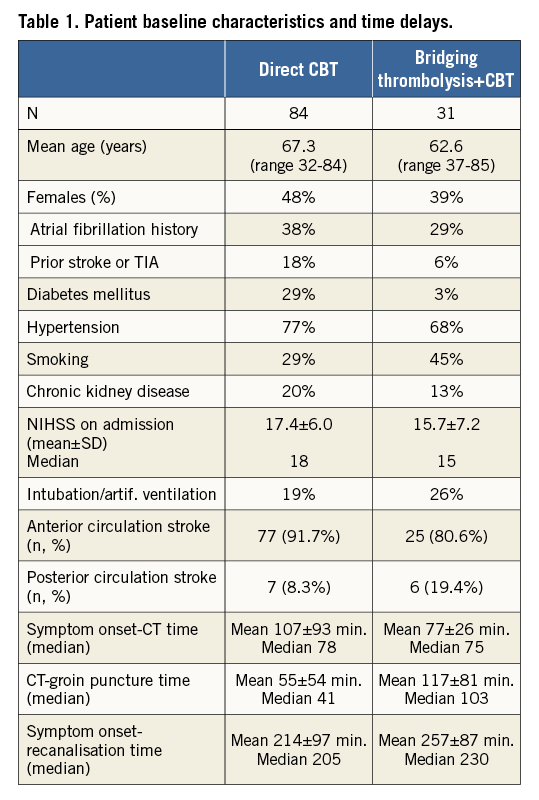
Patient baseline characteristics are shown in Table 1. A total of 115 patients were enrolled during a period of four years (October 2012 to September 2016). The number of procedures slowly increased during these four years: only 13 patients were enrolled during the first 12 months, 27 patients during the second year, 34 patients during the third year and 41 patients during the last 12 months. This increase mainly reflects the increased experience of the local neurologists, who became familiar with the indication to this procedure and increasingly trusted its results. Unfortunately, the referrals from the broader (secondary/tertiary) catchment area still remain very rare.
STATISTICS
Due to the above-mentioned biased decision to use bridging thrombolysis, we did not statistically compare direct CBT data versus bridging thrombolysis. Only the raw data are presented with basic descriptive statistics.
Results
TIME DELAYS
We carefully measured the time delays and these are listed in Table 1.
Angiographic outcomes are shown in Table 2. Isolated occlusion of the middle cerebral artery (MCA, segment M1 or rarely M2) was found in less than half of the patients (44%). Internal carotid artery occlusion or flow-limiting stenosis (extra- or intra-cranial segment) with or without simultaneous MCA occlusion was found in a similar proportion (44%) (Figure 2). The basilar artery was occluded in 10% and dominant vertebral artery in 1%. The overall angiographic success rate defined as TICI 2b/3 flow was 72% with differences between the occlusion sites (Table 2). While proximal ICA lesions were mostly atherosclerotic (only one patient had spontaneous carotid dissection without visible atherosclerosis), distal ICA and MCA lesions were embolic in all but one patient.
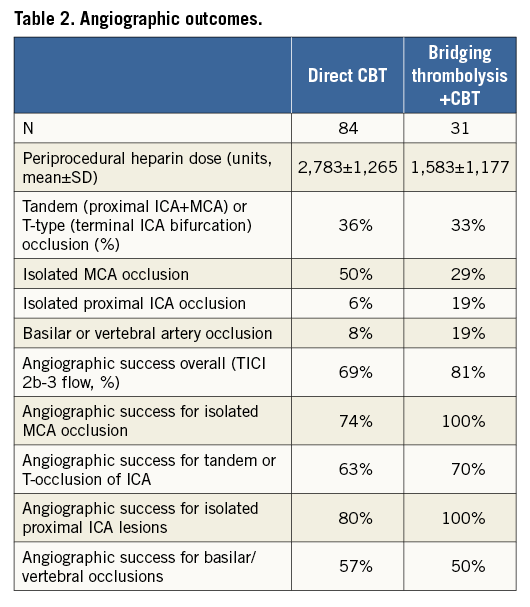
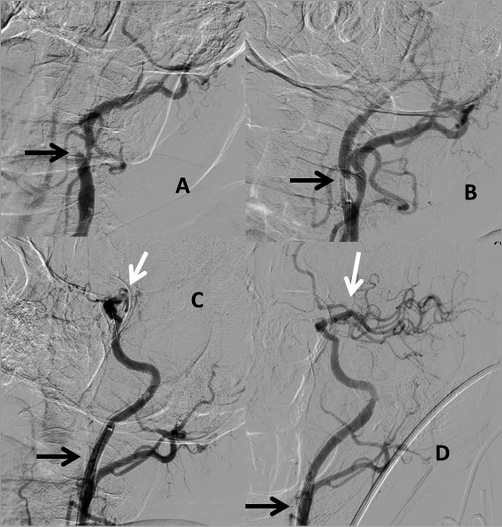
Figure 2. Severe (NIHSS=20) anterior circulation stroke caused by the tandem lesion: proximal occlusion of extracranial internal carotid artery (ICA) and distal “T” occlusion of intracranial ICA bifurcation. A) Angiography prior to the intervention showing a proximal ICA occlusion (black arrow). B) TIMI 1 flow in the ICA after guidewire passage, proximal critical lesion clearly visualised (black arrow). C) Proximal ICA after stenting (black arrow) and visualisation of intracranial “T” occlusion (white arrow). D) Final angiography showing a widely patent stented proximal ICA (black arrow) and normal flow through the ICA terminus to the intracranial branches (white arrow) after thrombus removal with a stent retriever.
CLINICAL OUTCOMES
Overall neurologic recovery (mRS ≤2 after 90 days) was achieved in 41% of patients. It was much higher (59%) in anterior circulation strokes with isolated occlusion of the middle cerebral artery (MCA) than in posterior (basilar/vertebral artery occlusions) strokes (25%). Clinical outcomes per treatment (direct CBT and bridging thrombolysis) are presented in Table 3. As explained above, these two groups are inherently different, thus no statistical comparisons can be made, but the data show some surprising trends: NIHSS improvement was faster in the direct CBT group but overall three-month outcomes are similar as was the risk of symptomatic intracranial bleeding (defined rigorously as any intracranial bleeding on imaging with NIHSS impairment of 4 or more).
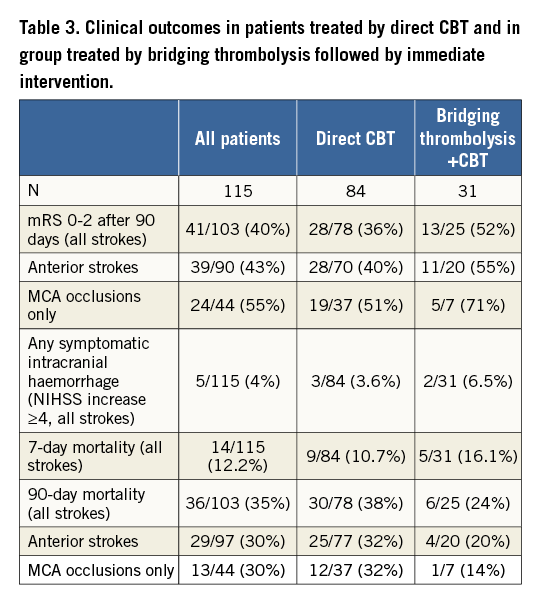
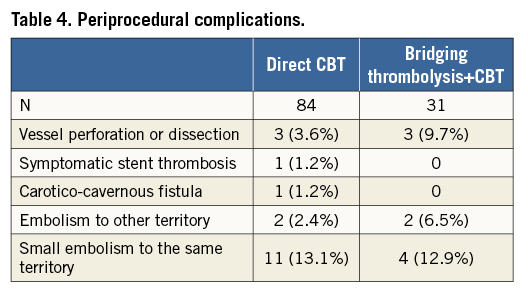
COMPLICATIONS
The full list of complications is shown in Table 4. The most frequent complication (intraparenchymal bleeding) was most likely not directly caused by catheter manipulations (no visible vessel rupture or contrast extravasation during the procedure), but rather by the fact that the artery was opened relatively late – and bleeding was reperfusion damage10.
Discussion
COMPARISON OF OUTCOMES WITH RANDOMISED TRIALS
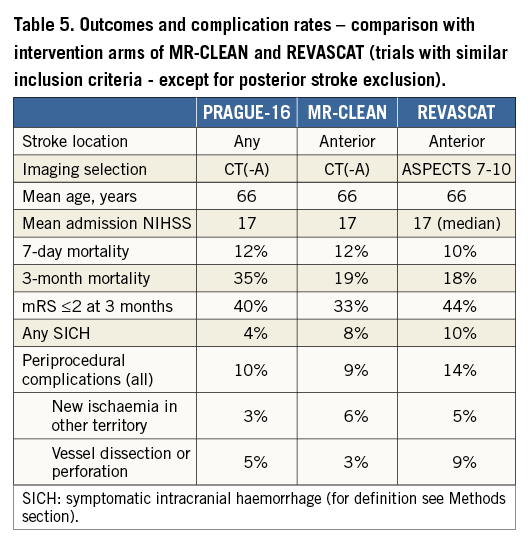
As our aim was to achieve a high standard in our performance, we compared our results with results of the expert neurointerventional centres from large randomised trials9-15. These seven trials enrolled 864 patients into the interventional arm (bridging thrombolysis in the majority of these patients) and 881 patients into the best medical (including thrombolysis whenever possible) therapy arm. Neurologic recovery (mRS 0-2 after three months) was achieved in 48% of patients treated by intervention versus 30% of patients treated by best medical therapy including thrombolysis. Our results (43% mRS 0-2 at 90 days among all anterior circulation strokes) did not achieve those of expert neurointerventional centres, but are clearly better then best medical therapy results in those trials. Also, angiographic outcomes (TICI 2b/3 flow 63-100% for various subgroups of anterior circulation strokes and 50-57% for posterior circulation strokes) can be compared with data published by expert neurointerventional centres. Thus, based on these results, the involvement of a cardiology department in acute stroke interventions may be beneficial for patients in places where expert neurointerventional radiology or endovascular neurosurgery is not available.
COMPLICATION RATES
Symptomatic intracranial haemorrhage was in the expected range (4%). Three-month mortality (30% for anterior circulation strokes) was numerically higher when compared with data from published randomised trials. This may be explained by the manner of patient selection (our approach was similar, e.g., to the MR CLEAN trial, while the EXTENT IA trial was more selective in terms of patient inclusion), by different baseline characteristics and very likely also by suboptimal rehabilitation after the acute phase in some patients transferred from the acute hospital to long-term facilities. Periprocedural complication rates (Table 4, Table 5) did not differ from the published data.
PERIPROCEDURAL HEPARIN USE
There are no data on periprocedural use of heparin in patients treated by direct intervention (without lytics). However, outside the interventional setting (with conservative treatment of ischaemic stroke), heparin is not indicated as it may increase the risk of haemorrhagic transformation. Thus, we empirically decided to use a very low dose of heparin in patients who did not receive bridging thrombolysis and no heparin in those with thrombolysis. Some neuro-operators do not even use any heparin during acute stroke interventions. The best strategy (heparin yes or no, heparin dose) is not known.
DIRECT CATHETER-BASED THROMBECTOMY VERSUS THROMBECTOMY AFTER BRIDGING THROMBOLYSIS
When the protocol for the PRAGUE-16 study was prepared and submitted to the local ethics committee (2012), we expected that direct CBT would be able to achieve superior outcomes to bridging thrombolysis followed by immediate CBT. The reality was different, and results in patients who received bridging thrombolysis were numerically better than in patients treated by direct CBT. As mentioned above, the baseline characteristics of these two groups are so different that it is not possible to draw any realistic conclusions: (1) bridging thrombolysis was used in situations when an immediate start of the intervention was not feasible (e.g., during the night) and thus the in-hospital time delay in the lytic group is double compared to the direct group, and (2) the lytic group was five years younger. The two groups are not comparable (inherently different), and any statistical comparison (even after adjustment) will be misleading, even more so since patient numbers are only moderate. Randomised trials are needed to compare direct mechanical thrombectomy versus bridging thrombolysis followed by thrombectomy. Nevertheless, almost all neurointerventional centres nowadays treat suitable patients with a contraindication for thrombolysis with mechanical thrombectomy although one study testing this question was prematurely terminated16.
REPERFUSION DAMAGE
While in acute MI the reperfusion damage is largely a theoretical consideration (because almost all AMI patients do benefit clinically from mechanical reperfusion), in acute ischaemic stroke the clinically apparent reperfusion damage is a real danger, with intraparenchymal bleeding seen in up to 10% of patients. This must be taken into consideration when deciding about an indication for intervention in acute ischaemic stroke.
ABSENCE OF ANGIOGRAPHICALLY VISIBLE INTRACRANIAL ATHEROSCLEROSIS
Another interesting difference when compared to acute coronary occlusion is the fact that intracranial occlusion of a large artery is almost always caused by an embolus and after thrombectomy the arteries do not show angiographic signs of atherosclerosis. Atherosclerotic lesions with in situ thrombosis are present typically only in patients with extracranial occlusions (proximal segment of the internal carotid artery or of the vertebral artery).
TRAINING OF INTERVENTIONALISTS
The 24/7 availability of endovascular interventions as routine treatment for emergent large vessel occlusion stroke varies across European countries: while in a few countries approximately 70-100 acute stroke interventions per million per year are carried out, in others this number is as low as five per million per year. The estimated need with current indications is 250-300 per million per year (authors’ own estimate based on unpublished stroke registry data from the Czech Republic). Thus, it is unclear how to organise the service in order to offer this effective treatment to the majority of European citizens. One possible way might be to train experienced interventional cardiologists. There are a few places where cardiologists already carry out this service17,18. Our centre started this programme by inviting one experienced interventional radiologist (B. Koznar) to join the high-volume interventional cardiology team. One interventional angiologist and one interventional cardiologist joined him in performing acute stroke interventions and thus are learning continuously. This may be an ideal scenario, but may be difficult to organise in other places. The only possible solution to this situation is a positive, open multidisciplinary cooperation at national or regional level based on the training requirements of multiple societies19.
Study limitations
The main limitation is the lack of randomised comparison. As this study is a prospective registry and patient allocation to d-CBT or TL-CBT groups was carried out by the attending neurologist based on the immediate availability of the intervention, the two groups cannot be compared directly by standard statistics. However, this study reflects a real-life scenario as no stroke patients undergoing intervention were excluded.
Conclusions
Direct catheter-based thrombectomy may be considered in patients with contraindications for TL or in patients with very short CT-groin puncture times, but bridging thrombolysis remains an important part of the treatment strategy. A randomised trial is needed to evaluate better the role of direct catheter-based thrombectomy. Acute stroke interventions performed in close cooperation among cardiologists, neurologists and radiologists have been proved to be feasible and safe.
| Impact on daily practice Catheter-based thrombectomy can be performed safely with good results within a cardiology department when smooth cooperation with local neurologists and radiologists is established and no specialised neuroradiology interventional service is available. Direct mechanical thrombectomy may be a reasonable alternative for patients with contraindications to thrombolysis or with very short CT-groin puncture times. |
Funding
Univerzita Karlova v Praze, project nr. P35.
Conflict of interest statement
The authors have no conflicts of interest to declare.

