Abstract
Aims: To assess the feasibility of direct catheter-based thrombectomy (d-CBT) performed jointly by cardiologists, neurologists and radiologists.
Methods and results: Computed tomography (CT) was completed within <6 hours from onset of acute ischaemic stroke and excluded bleeding or developed ischaemia in 23 patients who fulfilled pre-specified entry criteria. The mean NIHSS was 17 (8-24). Mechanical recanalisation was successful in 19/23 patients (83%). The mean symptom onset – CT time was 81 min, CT – sheath insertion 47 min, sheath – reperfusion 46 min. Three patients died within 30 days, two others within 90 days (overall three-month mortality 22%). The mean mRs at 90 days for the entire group was 3.19, among survivors 2.31 and among survivors treated within <120 minutes 1.17. Favourable functional outcome (mRs ≤2) was achieved in 48% of patients. Five patients (22%) had full (mRs=0) or nearly full (mRs=1) neurologic recovery. Seven patients were able to be discharged from neurology ICU directly home after a short (<7 days) hospital stay. Two patients had symptomatic intracranial haemorrhage.
Conclusions: Acute stroke treatment by d-CBT jointly by neurologists, cardiologists and radiologists provided promising results especially in patients reaching the cathlab within <2 hours from stroke onset.
Introduction
Direct mechanical reperfusion using catheter-based thrombectomy without thrombolysis (d-CBT) was first used in 20011 and there is, as yet, no randomised trial completed to date comparing mechanical reperfusion (without thrombolysis) versus intravenous thrombolysis. Thus, the latest official guidelines2 do not yet recognise direct mechanical intervention as the accepted routine therapy for acute stroke.
While in-hospital mortality of acute myocardial infarction has been successfully decreased to a current level of 5-8%, mortality on account of acute stroke has remained almost unchanged. Thrombolysis has not been associated with reductions in mortality due to acute ischaemic stroke. After having fully developed ST elevation myocardial infarction (STEMI) networks in their regions, many cardiologists worldwide are increasingly interested in acute stroke treatment. However, the interventional treatment of acute stroke (unlike acute myocardial infarction) requires effective cooperation among several medical specialities. The leading neurologists, neurosurgeons and neuroradiologists recognise the possibilities of effective regional STEMI networks (enabling 24/7 service for acute interventions) and are opening their minds to future cooperation with cardiologists to improve patient access to this modern therapy.
The current evidence for a non-facilitated (without thrombolysis) primary mechanical revascularisation in acute ischaemic stroke is limited – there are no randomised trials comparing mechanical reperfusion alone versus pharmacologic reperfusion alone. All published trials are spoiled by combined use of both approaches, despite the fact that no randomised study has demonstrated the benefit of such a combination over simple intravenous thrombolysis. Even the published registries of CBT include a mixture of patients treated with facilitated intervention and with direct mechanical intervention. A multidisciplinary approach using the existing “fast track” for acute myocardial infarction patients might be beneficial if it leads to shortening of unacceptably long time delays between the first medical contact and catheter intervention. We consider it absurd that in acute myocardial infarction the recommended time for door-to-sheath (needle) is 30 minutes, while in acute stroke (with a much shorter time window for reperfusion treatment due to the faster progression of irreversible brain damage) it is 60 or even 90 minutes. Therefore we tested a multidisciplinary (neurology+ radiology+cardiology) approach.
The aim of this pilot study was to assess the feasibility of d-CBT performed within a STEMI network in a multidisciplinary setting (close cooperation among cardiologists, neurologists and radiologists) in a single centre with no previously existing programme of acute stroke interventions.
Patients and methods
Patients were eligible if initial computed tomography (CT) demonstrated cerebral artery occlusion within <6 hours from onset of typical symptoms of an at least moderate (NIHSS ≥8) acute stroke, and if invasive angiography could be initiated after CT without delay. Exclusion criteria were: previously known moderate-severe neurologic symptoms, known coagulation disorders, known severe hypoglycaemia, intracranial bleeding (including in the history) and CT evidence of developed large ischaemia. The two co-primary endpoints of the study were 1) angiographic recanalisation rate and 2) functional neurologic outcome (mRs) at three months. Secondary endpoints were ΔNIHSS (admission–discharge), overall mortality at 30 and 90 days and symptomatic intracranial bleeding (defined as ΔNIHSS ≥4). The 90-day outcomes were assessed by board certified neurologists who were (as per study design) not blinded to the treatment.
During the initial study period of 16 months (10/2012 to 1/2014) only 23 patients (out of 540 stroke patients admitted to this hospital during the same period) fullfilled the entry criteria (Table 1). The two main reasons for non-inclusion to this pilot study were smaller stroke size (NIHSS <8) and late (>6 hours) presentation. The mean age was 64.8 years (range 32-84), 48% were females, and all patients had an anterior stroke. The mean admission NIHSS was 17 (range 8-24). Two patients suffered acute stroke while hospitalised for other illnesses in our hospital and 21 were brought to our hospital by the emergency medical system (EMS) ambulance. The EMS informed the neurologist on duty during the transport, brought the patient to the CT room and the neurologist usually met the patient on the way to the CT room.
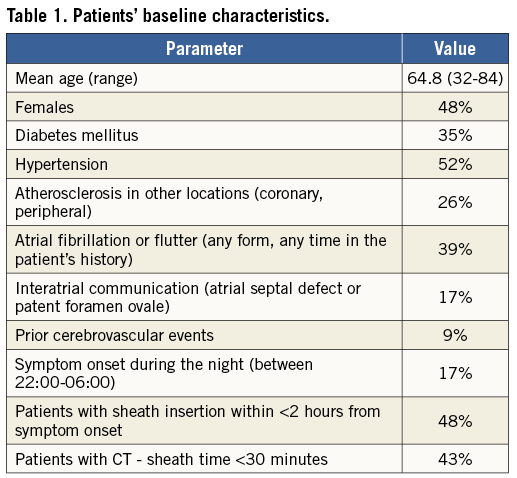
The primary strategy was to perform direct (primary) mechanical reperfusion (without lytics). Nevertheless, five patients received thrombolysis prior to mechanical intervention, and the remaining 18 patients received periprocedural heparin (mean dose 2,978 units, range 30-60 units/kg). The study protocol (specifically including abandoning of thrombolytics) was approved by the local ethical committee. The ethical committee recommended that, whenever written informed consent could not be signed by the patient (due to his/her actual mental status in the acute phase of stroke), it should be signed either by a close relative (if present) or by the indicating neurologist (who decided to perform the procedure).
INTERVENTIONS AND LOGISTICS
All patients were included based on the decision of a neurologist, who was present during the entry CT examination (Figure 1). After CT, patients were transferred to the catheterisation laboratory of a tertiary cardiac centre and intervention was performed jointly by an interventional radiologist (B.K.) and an interventional cardiologist (P.W.) via femoral access. Due to the low number of suitable patients (mean 1.5 patients per month) we decided not to involve more interventional specialists in acute stroke interventions in order to build and keep sufficient practical experience in those who performed the procedures. This strategy caused intervention unavailability in only one single case during the 16-month period, and five interventions were performed solely by the interventional radiologist (when P.W. was not available). The stent retrievers used were Trevo® (n=15) (Concentric Medical, Fremont, CA, USA), Solitaire® (n=10) (ev3, Plymouth, MN, USA), and Penumbra (n=3) (Penumbra Inc., Alameda, CA, USA). In 18 patients a single stent retriever was used, and in five patients two stent retrievers were used. In four patients simultaneous carotid intervention was performed with the use of the Wallstent® (Boston Scentific, Natick, MA, USA).
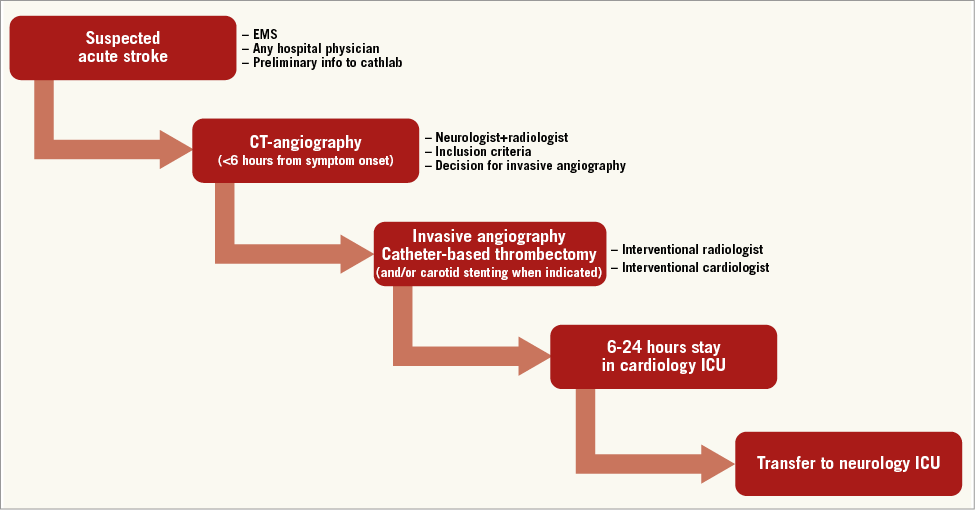
Figure 1. The logistics: sequence of actions in each patient with involved physicians’ specialisations. CT: computed tomography; EMS: emergency medical service; ICU: intensive care unit
Patients were admitted by a neurologist via CT and cathlab to a cardiology intensive care unit (ICU) and were transferred to a neurology ICU 12-24 hours after the intervention. The cardiology ICU was used for the initial hours after intervention for logistical reasons (initial set-up phase of an acute stroke interventional programme, neurology ICU in another building within the hospital).
GENERAL ANAESTHESIA
The default strategy was to perform the intervention without anaesthesia/intubation. Intubation was requested by the interventional radiologist in six patients (26%) due to persisting severe unrest. In these cases, an anaesthesiologist or an intensivist was called during the intervention from the cardiology ICU or from the anaesthesiology ICU to perform the intubation.
Results
ANGIOGRAPHIC FINDINGS
Medial cerebral artery (MCA) alone was occluded in 13 patients, internal carotid artery (ICA)+MCA in four patients, ICA+MCA+ anterior cerebral artery (ACA) in three, MCA+ACA in two and ICA alone in one patient. The 0.010” or 0.014” guidewire crossed the occlusion in 22/23 patients (96%). Mechanical recanalisation was successful in 19/23 patients (83%) after a mean of three thrombus retrieval attempts.
TIME DELAYS
The mean time delays (Table 2) were: symptom onset – CT 81 min, CT – arterial sheath insertion 47 min, sheath – reperfusion 46 min. Patients with symptom onset – cathlab time <2 hours had favourable outcome (mRs 0-2) in 55% versus 40% among those with time delay >2 hours.

CLINICAL OUTCOMES
Three patients (13%) died within 30 days, and two others within 90 days (overall three-month mortality 22%). The mean mRs at 90 days for the entire group was 3.19, and among survivors it was 2.31 (Figure 2). Favourable functional outcome (mRs ≤2) was achieved in 48% of patients. Of note, five patients (22%) had full (mRs=0) or nearly full (mRs=1) neurologic recovery. Seven patients were discharged from neurology ICU directly home after a short (<7 days) hospital stay, while 13 patients were transferred to rehabilitation units for further treatment. Two patients had symptomatic intracranial haemorrhage (NIHSS increase ≥4): one of them received thrombolysis. One patient had a smaller intracranial haemorrhage.
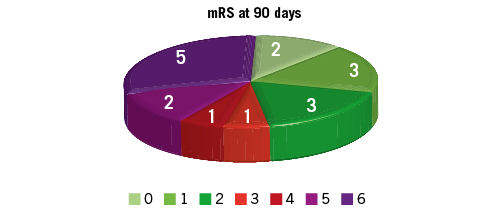
Figure 2. Functional outcomes as assessed by the modified Rankin scale (mRs) at 90 days. Green colour: good functional outcomes (mRs 0, 1, 2); red/violet colour: poor functional outcomes (mRs 3, 4, 5, 6).
OUTCOMES OF PATIENTS WITH INTERVENTION PERFORMED UNDER GENERAL ANAESTHESIA
The mean age of these six patients was 65 years (median 72). The mean admission NIHSS was 18.5 (range 14-24). Four of the six intubated patients died during the hospital stay, one remained severely limited (mRs=4) and one (32-year-old female with NIHSS 14 and with patent foramen ovale) fully recovered within 24 hours (mRs=0).
Discussion
POTENTIAL PRACTICAL IMPLICATIONS OF THIS STUDY
There are two key elements of this study which make it different from the previously published non-randomised trials: (a) default strategy for d-CBT, i.e., not to use thrombolytics, and (b) involvement of the cardiology catheterisation laboratory and the cardiology team jointly with an experienced neuroradiologist in order to minimise the time delays. In practical terms: 1) the cardiology nurse (who is present in the cathlab for acute myocardial infarction patients on the basis of 24/7 service) immediately after a phone alert initiates the cathlab equipment and prepares the materials for the procedure. Thus, the patient arrives from CT to a prepared cathlab, and 2) the interventional cardiologist may arrive before the radiologist and can start the procedure with the radiologist joining him a few minutes later.
TIME DELAYS
The time delays achieved in this study compare favourably with delays reported in most published studies. However, these delays could be considered unacceptable, leading one to postulate that an acceptable aim for the future is to achieve a mean CT – sheath time of around 30 minutes. Our group has considered modifying radically the patient flow to mirror the flow in acute myocardial infarction, i.e., to accept patients with an early (<2 hours from onset) and typical (hemiparesis/hemiplegia) acute stroke presentation directly to the cathlab. Such an approach might shorten the delays to reperfusion by at least 30 minutes. However, after discussions, the group elected to maintain the current patient flow via CT first. Future cathlabs equipped with combined CT/flat panel angiography could facilitate a reconsideration of the patient flow for acute stroke patients. The critical importance of time was recently reviewed by Sun et al3.
COMPARISON WITH RECENT LARGE RANDOMISED TRIALS
The results of this pilot study compare favourably with the endovascular arms of three recently published major randomised trials (IMS III, MR Rescue, SYNTHESIS)4-6, and in particular (Figure 3): similar 90-day mortality (19-22%, lower mortality in the SYNTHESIS trial was related to “softer” inclusion criteria allowing NIHSS ≥2), similar three-month functional outcomes (good outcome with mRs ≤2 in 41-48%, the mean 90-day mRs ranging between 2.9 and 3.9). The Interventional Management of Stroke (IMS III) trial4 has suspended enrolment due to futility. A major limitation of the IMS III trial was that patients were selected on clinical grounds and only 47% had a CT angiogram (CTA). In a preplanned analysis of the patients with a documented arterial occlusion by CTA there was a significant benefit in favour of facilitated intervention (p=0.01). These trial data on combined pharmacoinvasive therapy demonstrate that there is no benefit from facilitated intervention (iv thrombolysis followed by ia thrombolysis±catheter intervention) over iv thrombolysis alone in acute stroke.

Figure 3. Comparison of results of this pilot study with the endovascular treatment arms of the three largest randomised trials10-12.
MODERN STENT RETRIEVERS AND DIRECT CATHETER-BASED THROMBECTOMY
Evidence from large randomised trials with d-CBT is still lacking in acute ischaemic stroke. In the last three to five years several new clot retrieval devices (stent retrievers) have been introduced and received the CE mark for use in European patients. These devices (e.g., Solitaire or Trevo) could be considered to be a hybrid between a self-expanding stent and a soft “spider-web-like” basket for clot removal, and the risk of complications with these latest-generation stent retrievers is much smaller, while their success rates are higher. The published trials on the use of stent retrievers7-11 have demonstrated their feasibility and high recanalisation rates, but have no relevant implication to the question of whether reperfusion should be performed by mechanical means alone, pharmacological means alone or by a combined approach. The Penumbra pivotal stroke trial7 included 125 patients mostly pretreated by thrombolysis. The Solitaire With the Intention For Thrombectomy (SWIFT) trial8 testing the Solitaire stent retriever showed its superiority over an older predecessor, but also included a mixture of patients treated with and without thrombolysis. The TREVO 2 trial9 was similar to SWIFT and tested the Trevo stent retriever. In a single-centre study10 with the Solitaire stent retriever 75% of patients received thrombolysis before/during the intervention. A recent large multicentre retrospective review11 included 237 patients with acute anterior circulation occlusion, in whom endovascular treatment was initiated >8 hours (mean 15 hours) from the time last seen well. Successful revascularisation was achieved in 74%. Parenchymal haematoma occurred in 9%. The 90-day mortality rate was 21.5%, and there was an unfavourable outcome (mRs 3-6) in 55%.
The most recent meta-analysis12 of CBT registries identified 16 eligible published studies. Successful recanalisation was achieved in 59.1% (Merci), 86.6% (Penumbra) and 92.9% (stent retrievers). Functional independence (mRs ≤2) was achieved in 31.5% (Merci), 36.6% (Penumbra) and 46.9% (stent retrievers). The three-month mortality rate was 37.8% in the Merci studies, 20.7% in the Penumbra studies, and 12.3% in stent retriever studies. This study demonstrated improved outcomes after CBT when performed with the latest generation of stent retrievers. A major limitation of this and any other meta-analysis or comparison between stroke trials is the heterogeneity of the stroke patients enrolled and the criteria for patient selection. This heterogeneity stems from the multitude of causes of ischaemic stroke (e.g., atherosclerotic occlusion, cardioembolism, spontaneous dissection, etc.) as well as the variable sizes and locations of thrombi and occlusions. In addition, the status of collaterals, the severity of the ischaemic penumbra, and the size of the ischaemic core pretreatment all have an effect on prognosis and outcomes.
THE USE OF GENERAL ANAESTHESIA
Interventional techniques and periprocedural management are highly variable. Patients undergoing catheter-based interventions for acute ischaemic stroke receive either general anaesthesia (GA) or conscious sedation. GA may delay time to treatment, whereas conscious sedation may result in patient movement and compromise the safety of the procedure. Analysis of 980 patients who underwent intervention for acute anterior circulation stroke at 12 stroke centres between 2005 and 2009 found an overall recanalisation rate of 68% and symptomatic haemorrhage rate of 9.2%. GA was used in 44% of patients with no differences in intracranial haemorrhage rates when compared with the conscious sedation group. The use of GA was associated with poorer neurologic outcome at 90 days (odds ratio=2.33; 95% CI: 1.63-3.44; p<0.0001) and higher mortality (odds ratio=1.68; 95% CI: 1.23-2.30; p<0.0001) compared with conscious sedation. For example, it is becoming increasingly more likely that the use of general anaesthesia has a significant deleterious effect on outcomes and an increased mortality13,14.
INTERNAL CAROTID OCCLUSIONS
A recent study15 demonstrated that even stroke caused by the acute occlusion of the internal carotid artery (with only 8-17% recanalisation rate and 55% mortality rate when treated by thrombolysis) can be effectively treated by CBT: successful revascularisation of the extracranial internal carotid artery with acute stent implantation was achieved in 95% of patients. Intracranial recanalisation was achieved in 61% of patients who had simultaneous intracranial artery occlusion. The mortality rate was 13.6% at 90 days and there was a favourable outcome (mRs ≤2) in 41%.
Study limitations
This study has several important limitations. It is a small single-centre study. Although our centre has extensive experience in the routine treatment of all acute myocardial infarction patients with primary PCI, our centre has no prior experience in interventional treatment of acute stroke. The interventional radiologist involved (B.K.) had some experience in acute stroke interventions from his previous hospital. The absence of a randomised design and thus the absence of a control group is an obvious limitation. The study was designed with the expectation of 50-100 acute stroke interventions per year. The inclusion was lower and impacted on staff training. This issue is commonly faced in most other acute stroke centres in Europe – an inclusion of 10-50 acute stroke interventions per year is not unusual. The discussion as to why only a small proportion of acute stroke patients is suitable for CBT is beyond the scope of this paper, but two main reasons should be mentioned in brief: (a) most stroke patients arrive at the hospital late (when reperfusion would be rather deleterious), and (b) only about 30-40% of acute ischaemic stroke patients have a major cerebral artery (e.g., medial cerebral artery, basilar artery or internal carotid artery) occlusion demonstrated on the CT. Another limitation of our study is that we did not systematically measure final infarct volumes, which may be an interesting surrogate endpoint for similar small studies and which may have an important impact on patient outcomes16.
Future improvements
Different reperfusion methods in acute stroke compared to their use in acute myocardial infarction were described recently in a comprehensive review written jointly by interventional cardiologists and interventional neurologists17. We believe that d-CBT might be the best currently available treatment for acute ischaemic stroke, but only if a “military-like” organisation and “ultra-fast” patient flow through the system of medical care can be achieved. There are several options for shortening the delays: 1) the cathlab and the interventional specialist (radiologist, cardiologist or any other specialist with sufficient experience with acute vascular interventions in life-threatening situations) should be alerted as soon as the diagnosis of acute stroke is suspected (before CT scan!) even if this results in a few unnecessary alerts (no intervention indicated after CT scan) and a futile journey to the cathlab; 2) CT scanner and flat panel angiograph may be combined in one setting (ideally in the cathlab) to allow direct patient admission from the EMS ambulance to cathlab as is routinely done in acute myocardial infarction; and 3) the use of existing STEMI services may shorten the time delays (staff are in the hospital, cathlab can be prepared in a few minutes, cardiology centres have several cathlabs, thus at any time one can be free and ready immediately, etc.). It is likely that d-CBT (without thrombolysis) will be a preferred option in future for those patients with acute ischaemic stroke in whom the intervention can be started almost immediately (e.g., within <30 minutes) after CT scan, while the facilitated approach (intravenous thrombolysis directly at the CT room followed by CBT) may be good for situations with longer delays between CT and cathlab (e.g., transferred patients).
Thus, despite the limited experience (learning curve), the results of acute stroke treatment by direct catheter-based thrombectomy jointly by neurologists+cardiologists+radiologists are comparable to outcomes of patients randomised to endovascular therapy in the largest randomised trials. The latest generation of stent retrievers is able to recanalise 80-90% of occluded intracranial arteries – far more as compared to thrombolysis. However, it is not yet known whether this translates to better clinical outcomes. Sufficient data on outcomes after d-CBT (without thrombolysis) are still missing and trials comparing iv thrombolysis versus d-CBT are needed.
| Impact on daily practice Direct catheter-based thrombectomy is a promising reperfusion therapy for acute ischaemic stroke if it can be initiated within less than 45 minutes from CT scan. Collaboration of cardiologists, neurologists and radiologists offers the opportunity to shorten the time delays and thus to improve patient outcomes. If cardiology cathlab staff are present 24/7 on-site and are alerted even before computed tomography is done, the intervention can begin very soon after CT. Patients treated by this approach within <3 hours had excellent outcomes. The modern stent retrievers are very effective in achieving high recanalisation rates. Randomised trials are needed to show whether such fast-track direct catheter-based thrombectomy is more effective than intravenous thrombolysis. |
Acknowledgements
This study is supported by the Charles University projects P35, P34 and 260045/SVV/2014.
Conflict of interest statement
The authors have no conflicts of interest to declare.

