
Abstract
Aims: Our aim was to assess the effects on clinical outcomes of endovascular treatment vs. thrombolysis alone in patients with ischaemic stroke.
Methods and results: PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases were searched for randomised trials comparing endovascular treatment vs. intravenous thrombolysis alone in acute ischaemic stroke. Data were pooled by meta-analysis using a fixed-effects or a random-effects model, as appropriate. Eight studies enrolling 2,423 participants were included. Compared with thrombolysis alone, endovascular treatment was associated with higher rates of 90-day modified Rankin Scale (mRS) scores of 0-2 (42.4% vs. 31.8%, odds ratio [OR] 1.71, 95% confidence interval [CI]: 1.17-2.49, p=0.005, number needed to treat to benefit [NNTB]=8), and of recanalisation at 24-30 hours (76.9% vs. 39.6%, OR 4.49, 95% CI: 2.41-8.38, p<0.001, NNTB=2.9), with similar risk of symptomatic intracranial haemorrhage (5.4% vs. 4.9%, OR 1.08, 95% CI: 0.75-1.56, p=0.67) and all-cause death (15.3% vs. 16.6%, OR 0.86, 95% CI: 0.69-1.07, p=0.18). In subgroup analysis the benefits of endovascular treatment were restricted to studies where stent retriever systems were routinely employed.
Conclusions: In patients with acute ischaemic stroke, endovascular treatment is a safe and more effective strategy than intravenous thrombolysis alone.
Introduction
Acute ischaemic stroke is a frequently encountered disease associated with high mortality and morbidity in both Europe and the United States of America1,2. An intravenous tissue-type plasminogen activator (t-PA) given within 4.5 hours of symptom onset has been the mainstay of early treatment of acute ischaemic stroke3,4.
A more invasive strategy of intra-arterial thrombolysis plus low-dose heparin, as compared with low-dose intravenous heparin alone, has been shown to increase significantly the proportion of patients with a modified Rankin Scale (mRS) score of 0-2 at 90 days from 25% to 40%5. Unfortunately, subsequent randomised trials failed to show the superiority of endovascular treatment as compared with intravenous t-PA alone, thus raising questions about the utility of an interventional treatment6-8.
Very recently, new convincing data from five randomised trials have revealed different findings with regard to the clinical efficacy of endovascular treatment (i.e., thrombectomy following intravenous t-PA) as compared with intravenous t-PA alone9-13, leading to a change in recommendations for the treatment of acute ischaemic stroke14.
The aim of this study was to provide a comprehensive and quantitative assessment of evidence from early as well as contemporary randomised trials appraising the benefits and risks of endovascular treatment compared with intravenous t-PA alone in patients with acute ischaemic stroke.
Methods
DATA SOURCES AND SEARCH STRATEGY
A meta-analysis of randomised trials was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2009 guidelines15. Two reviewers (G. Ferrante and G.G. Stefanini) independently identified the relevant articles by an electronic search of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases (from inception to November 2015). The following search terms and keywords were used: “stroke, “endovascular treatment”, “endovascular therapy”, “intra-arterial fibrinolysis”, “intravenous thrombolysis”. No language, publication date, or publication status restrictions were imposed. All suitable non-published completed registered studies were considered for inclusion. We checked reference lists of identified articles, recent editorials, and related reviews.
STUDY SELECTION
Two reviewers (G. Ferrante and G.G. Stefanini) independently assessed trial eligibility based on titles, abstracts, full-text reports, and further information from investigators as needed. Discrepancies in study selection were resolved by consensus.
Eligible trials had to satisfy the following pre-specified criteria: 1) a randomised design that compared any endovascular treatment including intra-arterial fibrinolysis and/or first-generation mechanical embolectomy or newer stent retriever systems versus intravenous thrombolysis in patients with acute ischaemic stroke; 2) availability of mRS score at 90 days. Studies were excluded if trial results were available only as abstracts.
DATA EXTRACTION AND QUALITY ASSESSMENT
Two reviewers (G. Ferrante and G.G. Stefanini) independently extracted data (baseline characteristics, definition of outcomes, numbers of events) using a standardised data abstraction form, and independently and systematically assessed the studies’ methodological qualities using the Risk of Bias Assessment Tool from the Cochrane Handbook for randomised trials. This tool identifies selection, performance, attrition, detection, reporting bias, other sources of bias for each study, and classifies each of these as low, unclear, high16. A risk of bias summary reporting each risk of bias item for each included study was reported16. Disagreements were resolved via consensus between the two reviewers.
Data synthesis and data analysis
OUTCOME MEASURES
The primary efficacy endpoint was a 0-2 mRS score at 90 days as a measure of functional independence. The secondary efficacy endpoint was a successful recanalisation rate at 24 to 30 hours defined as Thrombolysis In Cerebral Infarction (TICI) angiographic scores of 2b (indicating successful reperfusion of ≥50%) or 3 (complete reperfusion).
The primary safety endpoint was symptomatic intracerebral haemorrhage; secondary safety endpoints were all-cause death, parenchymal haematoma, and subarachnoid haemorrhage. Endpoints were attributed according to the definition and timing used in each study.
Statistical analysis
The odds ratios (ORs) with 95% confidence interval (95% CI) for the endpoints were calculated from the available data. Trial-specific ORs were combined with the Mantel-Haenszel fixed-effects model or with the DerSimonian and Laird random-effects model if heterogeneity was statistically significant or I2 >25%17. The presence of heterogeneity among studies was evaluated with the Cochran’s Q chi-square test with p≤0.10 considered to be statistically significant, estimating the between-studies variance tau2, and using the I2 test to evaluate the inconsistency18.
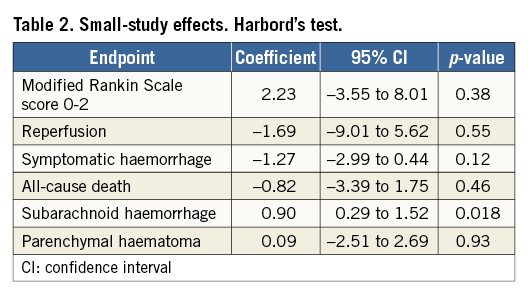
The number of patients needed to treat for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH), were calculated from meta-analytical estimates of pooled ORs, using the macro “metannt”, as 1/(projected control group event rate – projected treatment group event rate). The presence of small-study effects was investigated by using Harbord’s test, and by visual estimation with the use of contour-enhanced funnel plots19,20.
Subgroup analyses
Studies were divided into two subgroups according to the use of primarily intra-arterial fibrinolysis or first-generation mechanical embolectomy devices, alone or in combination (group 1), or the use of newer stent retriever systems (group 2).
An interaction test was used to assess the statistical significance of the difference between summary estimates of two subgroups as previously recommended21.
We also performed a random-effects meta-regression analysis to assess the impact of the use of newer stent retriever systems on treatment effect for the endpoints for which heterogeneity was found.
All analyses were conducted according to the intention-to-treat principle.
The statistical level of significance was two-tailed p<0.05. STATA 11.2 statistical software (StataCorp LP, College Station, TX, USA) and Review Manager (RevMan) [Computer program]. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011 - were used for statistical analyses.
Results
SEARCH RESULTS
Figure 1 shows the PRISMA flow diagram for study search and selection. Of the 5,179 citations screened, a total of eight randomised controlled trials comprising 2,423 patients with acute ischaemic stroke undergoing endovascular treatment were identified and included in this meta-analysis6-13.
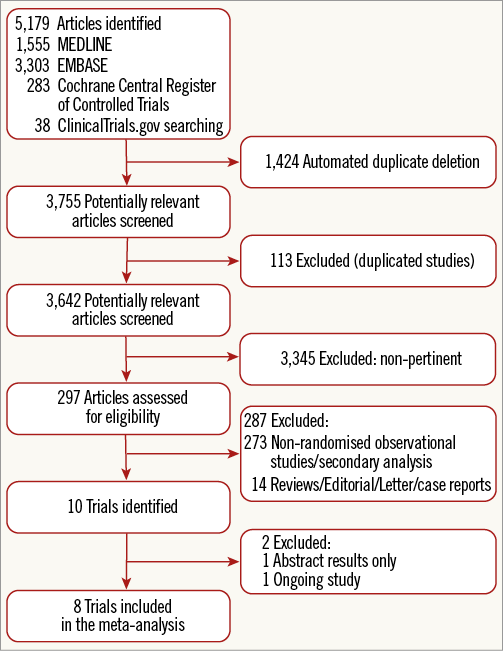
Figure 1. Flow diagram of the literature search for studies included in the meta-analysis according to the PRISMA statement.
STUDY CHARACTERISTICS
The main trial and patient characteristics of the included studies are shown in Table 1.
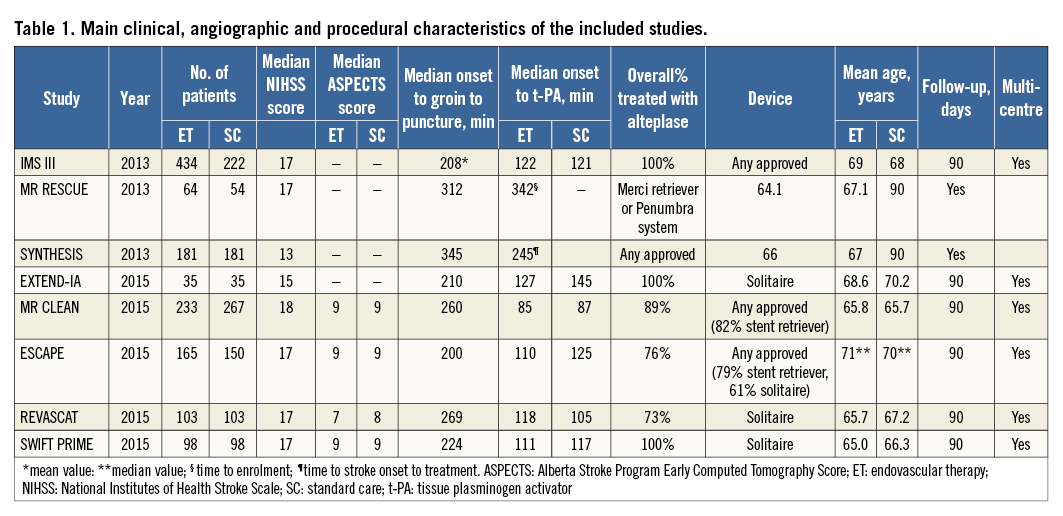
Three studies (IMS III6, MR RESCUE7, SYNTHESIS Expansion8), were carried out with primarily intra-arterial fibrinolysis or first-generation mechanical embolectomy devices, alone or in combination. These studies compared endovascular treatment over intravenous t-PA in intravenous t-PA eligible patients either as a substitute for initial treatment (SYNTHESIS Expansion8) or as subsequent intervention in those with persistent occlusion after intravenous t-PA (IMS III6, MR RESCUE7).
Five studies (EXTEND-IA, MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME)9-13 adopted systematic use of stent retrievable systems, with some studies permitting the use of salvage intra-arterial fibrinolytic drugs (i.e., MR CLEAN10, ESCAPE11, SWIFT PRIME13), while other studies did not allow this use (EXTEND-IA9, REVASCAT12).
In these five studies9-13 almost all patients received intravenous t-PA. In two trials (MR CLEAN10 and REVASCAT12) a waiting period of time after beginning the administration of intravenous t-PA before proceeding to endovascular treatment was present, whereas in the other three trials no waiting period was planned (ESCAPE11, SWIFT PRIME13, and EXTEND-IA9) .
Of the three studies6-8 with intra-arterial fibrinolysis or first-generation mechanical embolectomy, two studies6,8 enrolled patients within six hours from symptom onset, while MR RESCUE7 enrolled patients up to eight hours from symptom onset.
Of the five stent retriever studies, three studies specified a six-hour window from symptom onset9,10,13, while in two studies11,12 treatment was permitted up to eight to 12 hours after symptom onset.
In two studies6,8 no vascular imaging was used to assess the vascular territory responsible for occlusion. In three studies patients with occlusion of anterior circulation were selected7,9,10. In another three studies occlusions of the internal carotid artery/mid cerebral artery (any site or M1) were selected11-13.
BIAS AND SMALL-STUDY EFFECTS
Figure 2 summarises systematic bias assessment of the included studies. Overall, there was a high prevalence of low risk of bias for most domains across most studies. No evidence for small-study effect was detected by Harbord’s test for all endpoints except for subarachnoid haemorrhage (Table 2).

Figure 2. Risk of bias summary: judgements about each risk of bias item for each included study.
Contour-enhanced funnel plots showed asymmetry, probably due to publication bias based on statistical significance, for the endpoints of modified Rankin Scale score and recanalisation (Figure 3, Figure 4). Smaller asymmetry was also noted for symptomatic intracranial haemorrhage, all-cause death and subarachnoid haemorrhage (Figure 5-Figure 7); no asymmetry was found for parenchymal haematoma (Figure 8).
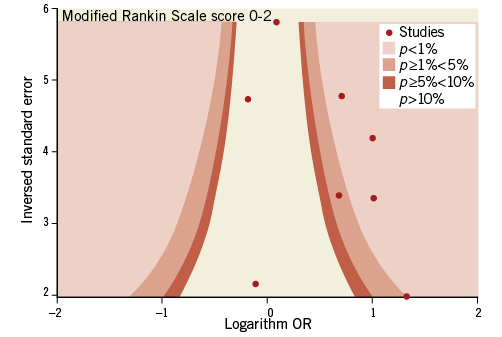
Figure 3. Contour-enhanced funnel plot for mRS score of 0-2.
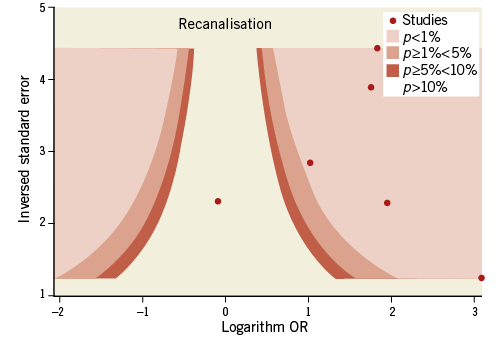
Figure 4. Contour-enhanced funnel plot for recanalisation.
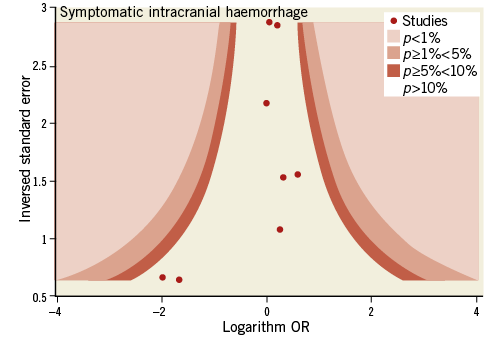
Figure 5. Contour-enhanced funnel plot for symptomatic intracranial haemorrhage.
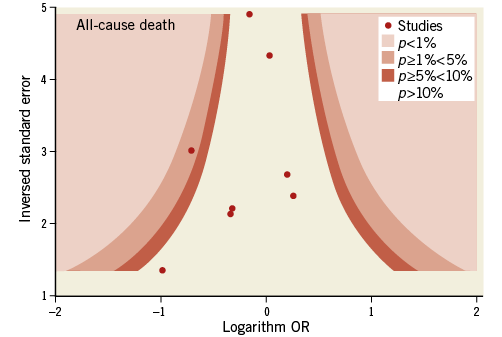
Figure 6. Contour-enhanced funnel plot for all-cause death.
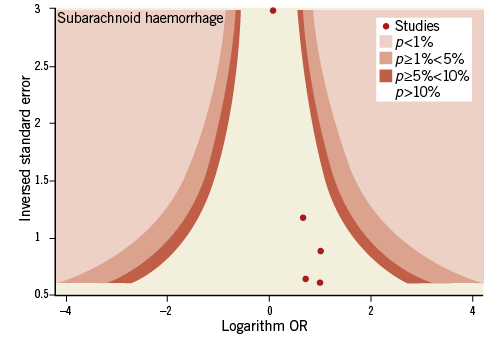
Figure 7. Contour-enhanced funnel plot for subarachnoid haemorrhage.
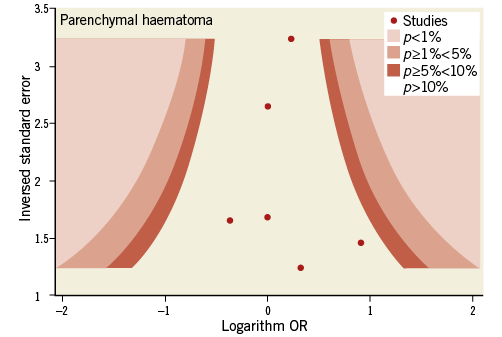
Figure 8. Contour-enhanced funnel plot for parenchymal haematoma.
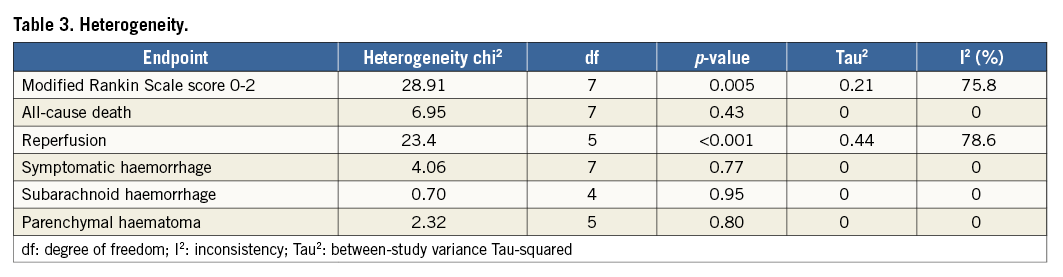
Heterogeneity
Significant heterogeneity was found for the endpoints of 90-day modified Rankin Scale score of 0-2 and recanalisation rate (Table 3). No evidence of heterogeneity was found for the remaining endpoints (Table 3).
Outcomes
Compared with t-PA alone, endovascular treatment was associated with higher rates of 90-day modified Rankin Scale score of 0-2 (42.4% vs. 31.8%, odds ratio [OR] 1.71, 95% confidence interval [CI] 1.17-2.49, p=0.005, number needed to treat to benefit [NNTB]=8), and of recanalisation at 24-30 hours (76.9% vs. 39.6%, OR 4.49, 95% CI: 2.41-8.38, p<0.001, NNTB=2.9) (Table 4, Figure 9, Figure 10). The rates of symptomatic intracranial haemorrhage and of parenchymal haemorrhage were similar in both groups (Table 4, Figure 11, Figure 12). No significant difference in all-cause mortality was observed between groups (Table 4, Figure 13). However, endovascular treatment was associated with an increased risk of subarachnoid haemorrhage (5.9% vs. 1.82%, OR 2.38, 95% CI: 1.35-4.20, p=0.003, NNTH=42) (Table 4, Figure 14).
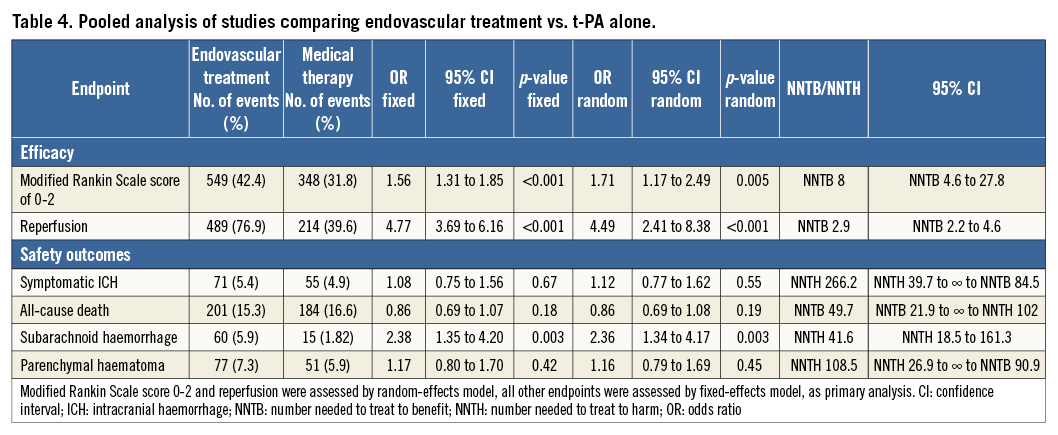
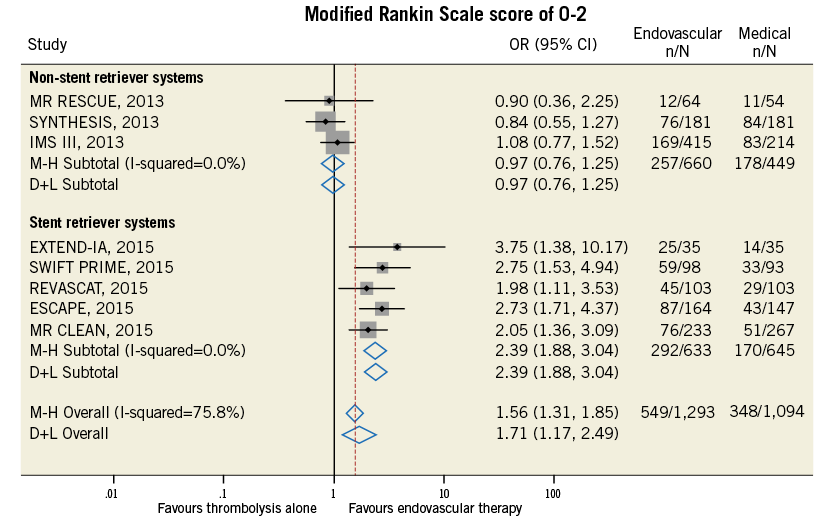
Figure 9. Forest plot reporting trial-specific and summary odds ratios (OR) with 95% confidence interval (CI) in the overall population and in subgroups according to the use of stent retriever systems for the endpoint of modified Rankin Scale score of 0-2 at 90 days. M-H: Mantel-Haenszel fixed-effects method; D+L: DerSimonian and Laird random-effects method
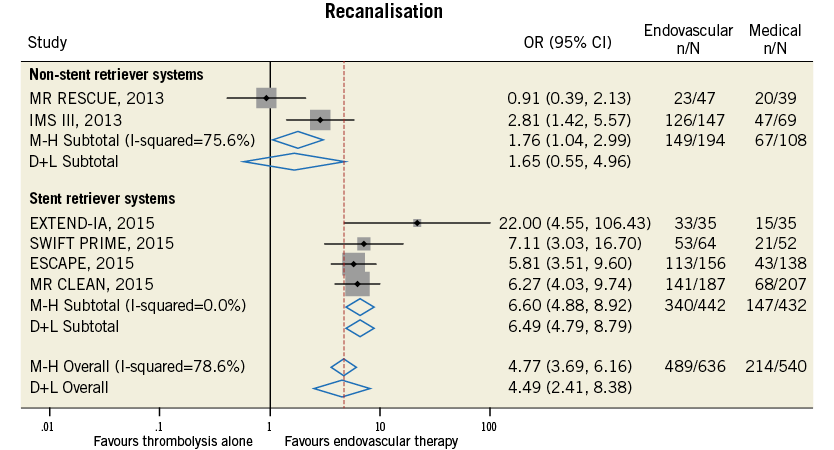
Figure 10. Forest plot for the endpoint of recanalisation rate assessed as modified TICI score of 2b-3.
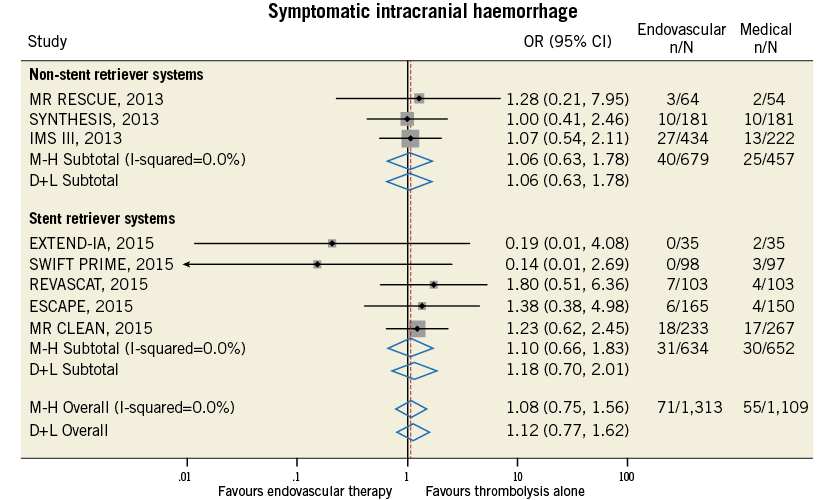
Figure 11. Forest plot for the endpoint of symptomatic intracranial haemorrhage.
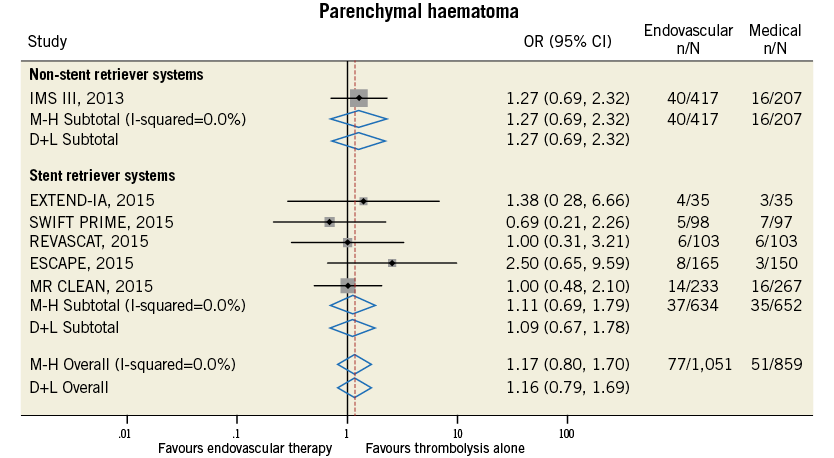
Figure 12. Forest plot for the endpoint of parenchymal haematoma.

Figure 13. Forest plot for the endpoint of all-cause death.
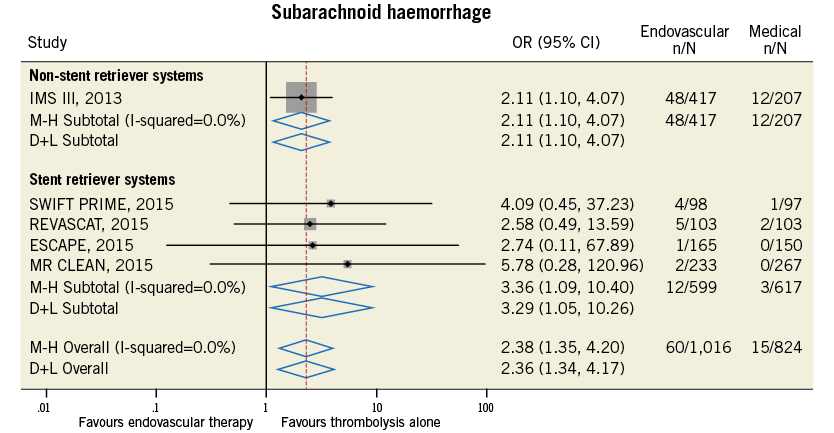
Figure 14. Forest plot for the endpoint of subarachnoid haemorrhage.
SUBGROUP ANALYSES
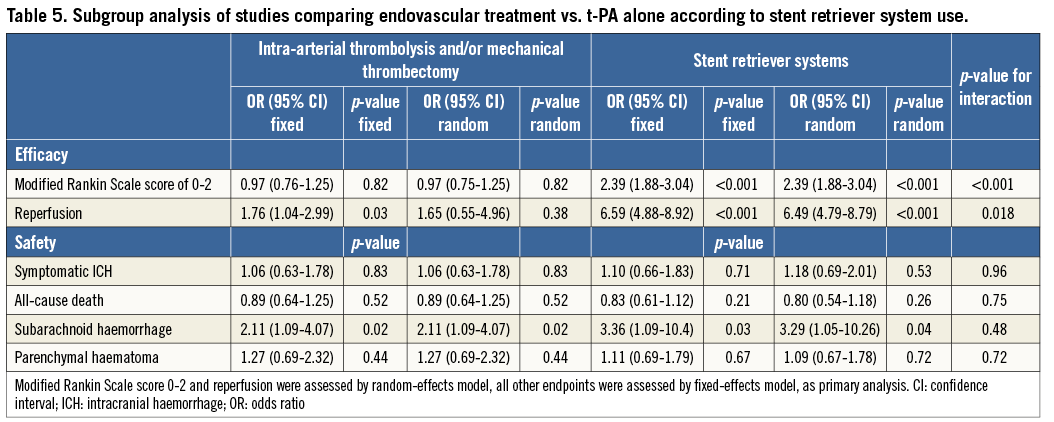
Endovascular treatment was effective in terms of improvement in functional independence at 90 days and of recanalisation rate at 24-30 hours in the subgroup of studies employing stent retriever systems only, while a neutral effect on outcomes was observed in non-stent retriever studies (p for interaction <0.05) (Table 5). The increase in symptomatic intracranial haemorrhage associated with endovascular treatment was observed in both subgroups (Table 5). Meta-regression analysis found that the use of stent retriever systems has a significant positive impact on treatment effect for the endpoint of functional independence at 90 days (OR 2.25, 95% CI: 1.59-3.79, p=0.002) and of recanalisation rate at 24-30 hours (OR 3.73, 95% CI: 1.16-12.06, p=0.035).
Discussion
The main findings of this meta-analysis including 2,423 patients with acute ischaemic stroke, the vast majority presenting with large proximal vessel occlusions, are the following:
1. Compared with t-PA alone, endovascular treatment is associated with a significant clinically relevant increase of functional independence at 90 days and of recanalisation rates at 24 to 30 hours. Of note, for every eight patients who were treated, one additional patient was functionally independent at 90-day follow-up, and, for every 2.9 patients who were treated, one additional patient had successful recanalisation at 24 to 30 hours.
2. Rates of symptomatic intracranial haemorrhage and of parenchymal haemorrhage were similar in both the endovascular and the thrombolytic alone group, with no difference in mortality between groups. However, a significantly increased risk of subarachnoid haemorrhage associated with endovascular treatment use was observed.
A pre-specified subgroup analysis found that the benefits of endovascular treatment on efficacy outcomes were realised only in studies where there was systematic use of stent retriever systems. These findings show that endovascular treatment is a safe and more effective strategy than intravenous t-PA alone for the treatment of acute ischaemic stroke.
Earlier studies
In the SYNTHESIS Expansion trial8, endovascular treatment was not found to be superior over t-PA, at 15 percentage points lower disability-free survival at 90 days, with freedom from disability defined as a modified Rankin Scale score of 0 or 1. In this trial, patients who were randomly assigned to the endovascular treatment group did not receive intravenous t-PA while awaiting endovascular treatment. After angiography, the choice between pharmacologic and mechanical thrombolysis or both was left to the interventionist. Furthermore, the demonstration of vessel occlusion was not a mandatory eligibility criterion.
The MR RESCUE trial7 assessed whether endovascular treatment could be superior to standard medical therapy among patients presenting with a substantial ischaemic penumbral tissue and a small volume of predicted core infarct. No significant outcome differences between treatment groups were found. However, imaging modalities for assessing penumbral tissue were heterogeneous, there was a large time delay between imaging and embolectomy, and a lower rate of revascularisation in the embolectomy group was finally achieved.
The IMS III trial6 was stopped early because of lack of differences between endovascular treatment and intravenous t-PA in terms of functional recovery in the overall population and across multiple subgroups. However, non-significant trends towards better outcomes with endovascular treatment, as compared to intravenous t-PA, were observed in patients within two hours after the onset of symptoms as well as among patients with a time from the start of intravenous t-PA to groin puncture of 90 minutes or less.
Recent studies
In contrast with these negative trials, an increase in the effectiveness of endovascular treatment, as compared to intravenous thrombolytic alone, has been consistently observed in randomised trials published in 2015, with higher benefits found in the EXTEND-IA9, ESCAPE11, and SWIFT PRIME13 studies, and lower effects observed in the MR CLEAN10 and REVASCAT12 studies.
Potential factors associated with this change in treatment effect are the use of stent-retriever device technology and a reduction in time delay between admission and groin puncture, leading to higher rates of recanalisation, and perhaps the use of neuroimaging modalities for documenting vessel occlusion and for patient selection. Indeed, in the EXTEND-IA trial9 among patients with proximal cerebral arterial occlusion and salvageable tissue on computed tomography perfusion imaging, endovascular treatment with the use of the Solitaire™ FR revascularisation device (stent retriever) (ev3/Covidien, Plymouth, MN, USA) was performed within a median of 93 minutes from initial neuroimaging assessment. In the ESCAPE trial11, retrievable stents were used in 86.1% of patients, the median time from computed tomography to first reperfusion was 84 minutes, and the median time from groin puncture to first reperfusion was 30 minutes. In the SWIFT PRIME trial13, a stent retriever was used in 89% of patients and a speedy endovascular therapy was performed with a median time of 90 minutes from hospital arrival to groin puncture. Of pivotal importance was that groin puncture and stent retriever deployment generally took place during t-PA infusion. Usage of retrievable stents was 82% in the MR CLEAN trial10, and 86.1% in the ESCAPE trial11. Stent-retriever device technology results have shown faster and more complete recanalisation. Our subgroup analysis showing that a benefit of endovascular treatment was restricted to studies with routine adoption of such technology supports this hypothesis. The lower magnitude of treatment effect seen in the MR CLEAN10 and REVASCAT12 trials could depend on longer times from hospital admission to reperfusion and lower reperfusion rates. Indeed, the reperfusion rate was 59% in the MR CLEAN trial10 and 66% in the REVASCAT trial12, as compared to 88% in the SWIFT PRIME trial13. In the REVASCAT trial12, only patients with a documented artery occlusion 30 minutes after alteplase administration were selected, thus delaying the initiation of endovascular treatment. Also, the inclusion of patients with larger infarct sizes in this trial could have played a role.
In more recent studies, the demonstration of large-vessel occlusion has become an eligibility criterion for patient selection9-13. Still, it remains controversial whether the determination of the volume of irreversibly infarcted brain tissue on admission is also required for patient eligibility for endovascular treatment. Further, there is no consensus as yet on which is the best neuroimaging criterion to be adopted for this purpose. In the EXTEND-IA9 and SWIFT PRIME13 trials, the ratio of ischaemic tissue at risk to irreversibly infarcted brain (i.e., penumbral mismatch) with varying cut-offs of 20% or 80%, respectively, was used. Also core-infarct volume with cut-offs of 70 ml or 50 ml was used to exclude patients in the EXTEND-IA9 and in the SWIFT PRIME13 trial, respectively. The Alberta Stroke Program Early CT Score (ASPECTS)22 was used as a measure of infarct core in the ESCAPE11 and REVASCAT12 trials, with exclusion of patients with an ASPECTS of less than six. The MR CLEAN trial10 required only vessel imaging for patient selection.
Clinical implications
The absolute benefit of endovascular treatment, as compared to intravenous thrombolysis alone, on functional independence at 90 days is estimated at a NNTB of 8 with a comparable safety profile in terms of symptomatic intracranial haemorrhage and mortality. Considering the higher incidence and morbidity of acute ischaemic stroke, the present findings prompt the development of a capillary and effective network of stroke care centres to adapt to endovascular therapy.
Collaboration among neuroradiologists, interventional cardiologists, and also vascular surgeons or neurosurgeons with expertise in acute endovascular procedures could probably allow building a 24-hour/seven-day active stroke care system similar to that for primary angioplasty for acute myocardial infarction.
Limitations
Our study has important limitations. First, it is not an individual patient data meta-analysis which could allow an assessment of the impact of selected variables on treatment effect, such as time from symptom onset to hospital admission, baseline extent of irreversible brain injury, thus allowing identification of the subgroups of patients which could benefit the most from endovascular treatment.
Second, the detection of significant heterogeneity for the endpoint of modified Rankin Scale score of 0-2 and recanalisation rates represents another limitation. Studies differed with regard to trial design, type of endovascular treatment, eligibility criteria for patient selection, imaging modalities for assessment of vessel occlusion or core infarct at baseline, and time from symptom onset to hospital admission.
Third, evidence of significant bias for the endpoints of modified Rankin Scale score and recanalisation as assessed by contour-enhanced funnel plot was found, as well as for the endpoint of subarachnoid haemorrhage by Harbord’s test.
Conclusions
This meta-analysis of eight randomised trials including 2,423 patients with acute ischaemic stroke, mostly with large proximal vessel occlusions, shows that endovascular treatment, as compared with t-PA alone, is a safe and more effective strategy, as it improves early reperfusion and functional independence at 90 days after stroke and is associated with a similar risk of symptomatic intracranial haemorrhage and mortality. These findings prompt a paradigm shift in the treatment of patients with acute ischaemic stroke.
| Impact on daily practice Endovascular treatment is an effective and safe way to treat acute ischaemic stroke and it improves patient outcomes. The absolute benefit of endovascular treatment on functional independence at 90 days is high and is estimated at a NNTB of 8. There is a need for a 24-hour/seven-day active stroke care system, similar to that for primary angioplasty for acute myocardial infarction, in order to deliver endovascular treatment rapidly. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

