Abstract
Background: The use of oversizing in mitral valve-in-valve (MViV) procedures can lead to non-uniform expansion of transcatheter heart valves (THV). This may have implications for THV durability.
Aims: The objective of this study was to assess the extent and predictors of THV deformation in MViV procedures.
Methods: We examined 33 patients who underwent MViV with SAPIEN prostheses. The extent of THV deformation (deformation index, eccentricity, neosinus volume, asymmetric leaflet expansion and vertical deformation) and hypoattenuating leaflet thickening (HALT) were assessed using cardiac computed tomography (CT), performed prospectively at 30 days post-procedure. For descriptive purposes, the THV deformation index was calculated, with values >1.00 representing a more hourglass shape.
Results: Non-uniform underexpansion of THV was common after MViV implantation, with a median expansion area of 74.0% (interquartile range 68.1-84.1) at the narrowest level and a THV deformation index of 1.21 (1.13-1.29), but circularity was maintained with eccentricity ranging from 0.24 to 0.28. The degree of oversizing was a key factor associated with greater underexpansion and a higher deformation index (β=−0.634; p<0.001; β=0.594; p<0.001, respectively). Overall, the incidence of HALT on the 30-day postprocedural CT was 27.3% (9 of 33). Most patients (32 of 33) were on anticoagulation therapy, but the prothrombin time and international normalised ratio (PT-INR) at the time of the CT scan was <2.5 in 23 of 32 patients. Among patients with a PT-INR of <2.5, HALT was predominantly observed with a high THV deformation index of ≥1.18.
Conclusions: THV deformation, i.e., underexpansion and an hourglass shape, commonly occurs after MViV implantation and is negatively affected by excessive oversizing. Optimising THV expansion during MViV could potentially prevent HALT.
Introduction
Transcatheter mitral valve-in-valve (MViV) implantation is an established therapeutic option for selected patients with surgical heart valve (SHV) degeneration, with demonstrated safety and effectiveness1234. While cases of MViV are commonly performed with balloon-expandable SAPIEN (Edwards Lifesciences) devices13 which were originally designed for the aortic position, the original intended design of this transcatheter heart valve (THV) prosthesis is to maintain a uniform, cylindrical shape for its ideal function.
During MViV procedures, oversizing the THV, with respect to the true internal diameter (True ID) of the SHV, and flaring of the outflow are used in order to ensure fixation and reduce the risk of embolisation156. This can result in a non-uniform underexpansion of the THV, with potential consequences of pinwheeling, shear stress on the leaflets, increased blood stasis and flow stagnation as shown in previous in vitro testing78910. This could affect THV durability. In a recent study, we observed that such prosthesis deformation was also related to an increased risk of hypoattenuating leaflet thickening (HALT) in patients treated with transcatheter aortic valve replacement (TAVR)11. In addition, adverse haemodynamic effects due to excessive underexpansion of the functional portion of the THV were observed after aortic valve-in-valve procedures12. Given these in vitro and in vivo findings, insight into the extent of THV deformation following MViV implantation and its relation to HALT and haemodynamics is important. This is particularly relevant as post-dilatation, with or without fracture, to optimise THV expansion is utilised less frequently in the current practice of MViV151314.
In our investigation, we performed the THV assessment with cardiac computed tomography (CT), with detailed examination of THV expansion and leaflet alignment to evaluate the extent and the predictors of THV deformation with MViV.
Methods
STUDY DESIGN AND PATIENT POPULATION
Starting on 1 July 2015, a prospective internal registry was initiated at the Minneapolis Heart Institute at Abbott Northwestern Hospital (Minneapolis, MN, USA) for patients who received THV implantation, whereby a retrospective electrocardiogram (ECG)-gated contrast-enhanced cardiac CT examination was performed to detect HALT and assess leaflet mobility at 30 days post-procedure. All patients were consented to participate unless there was a medical (e.g., high risk of contrast-induced nephropathy) or social (e.g., unable to travel) contraindication to the performance of the study, as previously described15. This current study included a subset of patients with mitral SHV degeneration who had been successfully treated with a ViV procedure using either a balloon-expandable SAPIEN 3 or 3 Ultra (S3/S3 Ultra) prosthesis (sizes 23, 26, or 29 mm) and who had undergone such CT examinations between 1 July 2015 and 31 January 2023. All patients underwent a comprehensive clinical evaluation by a Heart Team, and the MViV procedure was performed according to established procedural strategies, as previously shown1516. The transseptal approach was the preferred approach, and the transapical approach was used only in the earlier part of our experience. Selection of the THV size and use of pre-/postdilatation were left to the operator’s discretion. Patients who converted to surgery, had THV migration, needed a second THV implantation or who suffered perioperative death that precluded subsequent CT imaging were excluded.
The present study was conducted in accordance with the Declaration of Helsinki and approved by the Allina Institutional Review Board. All patients provided informed consent for the use of their medical records for research purposes.
INSTITUTIONAL ANTICOAGULATION PROTOCOL
Prior to the MViV procedure, patients on anticoagulation therapy discontinued anticoagulation for 4 days (for vitamin K antagonists [i.e., warfarin]) or for 48 hours (for direct oral anticoagulation). All patients routinely received a loading dose of aspirin (325 mg) on the day of the procedure. During the procedure, weight-adjusted unfractionated heparin was given to achieve an activated clotting time of ≥250 seconds. Following MViV implantation, patients received warfarin with an international normalised ratio (INR) goal of 2.5 − for the first 3 months with oral aspirin, followed by lifelong aspirin therapy. For patients with indications for long-term anticoagulation, a combination of warfarin or direct oral anticoagulation and single antiplatelet therapy for 3 to 6 months was administrated, followed by lifelong anticoagulation therapy based on the risks of thromboembolism and bleeding.
CT IMAGE ACQUISITION AND ANALYSIS
Contrast-enhanced ECG-gated cardiac CT was performed at 30 days post-MViV procedure using previously published acquisition protocols (Supplementary Appendix 1). All CT imaging analyses were performed on a dedicated post-processing workstation equipped with Circle cvi42 (Version 5.13.7; Circle Cardiovascular Imaging) by a single imaging core lab expert (M. Fukui) in a dedicated cardiac CT core laboratory.
IN VITRO THV ASSESSMENT
To evaluate the extent of S3/S3 Ultra prosthesis deformation compared to the design intent, we performed CT examinations of uncrimped, fully expanded 23, 26 and 29 mm SAPIEN 3 prostheses with the same scanner used in this study cohort. The external stent frame areas for the SAPIEN THV prostheses were measured at 2 levels: leaflet outflow (i.e., the 3 commissural tabs of the prosthetic leaflets) and leaflet inflow (i.e., the nadir of the prosthetic leaflets) (Figure 1) at nominal expansion. The nominal in vitro neosinus volume of each valve size was measured as the volume above the prosthesis leaflets within the prosthesis frame using a short stack of images reconstructed from the nadir to the tips of the THV, using a 1.0 mm slice thickness without a gap, following previously published protocols11.
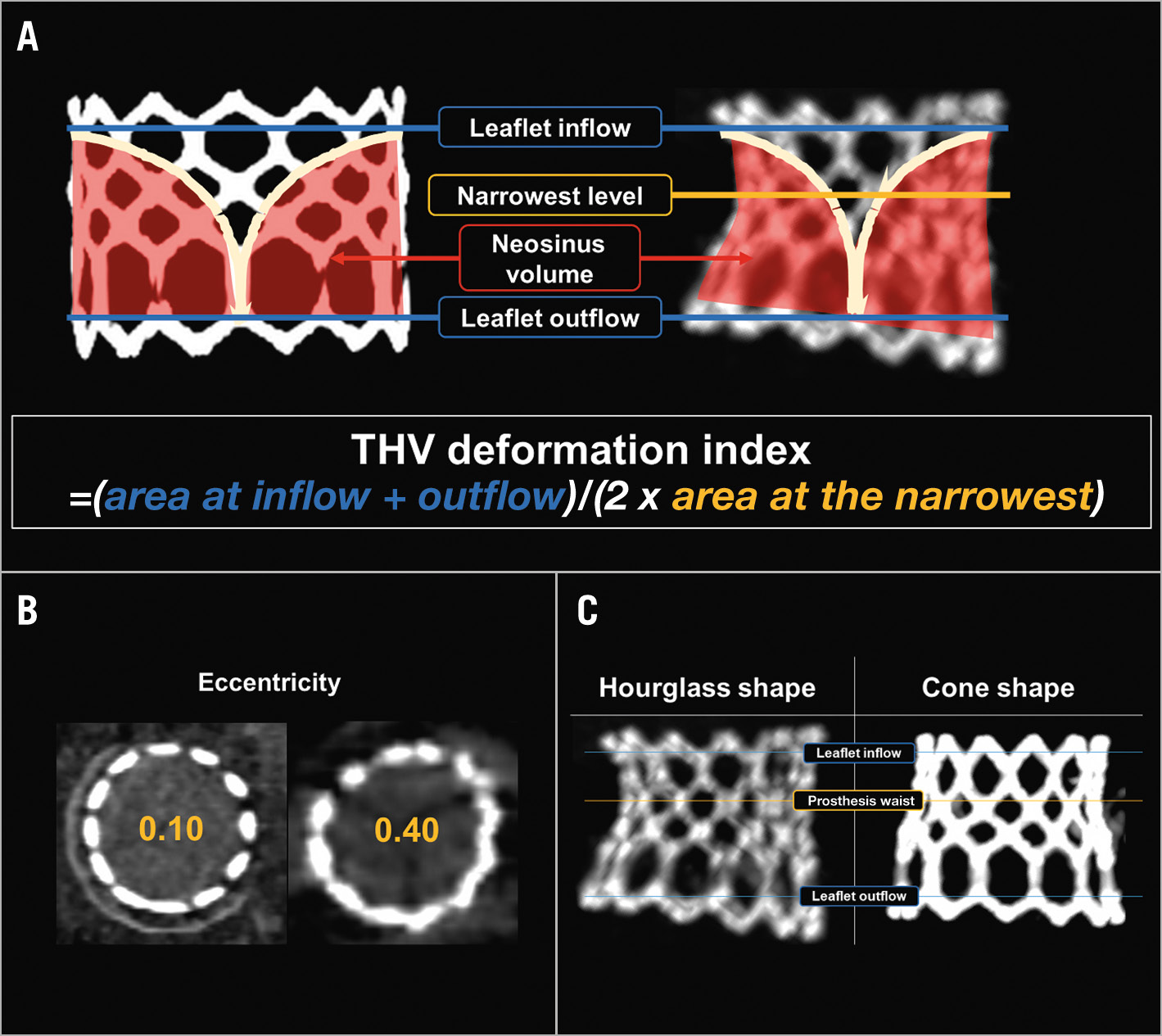
Figure 1. Assessment of transcatheter heart valve deformation. A,B) Transcatheter heart valve (THV) expansion area and eccentricity are assessed at 3 levels: leaflet inflow, the narrowest level and leaflet outflow. The THV deformation index is calculated using the expansion area at the 3 levels as (area at leaflet outflow+area at leaflet inflow)/(2×area at the narrowest level). The neosinus volume is measured as the volume above the prosthesis leaflet within the stent frame. C) The design intent of the SAPIEN prosthesis is a cylindrical shape with the same cross-sectional area throughout the frame (A, left). However, 2 distinct configurations of the S3/S3 Ultra frame were observed after MViV implantation; an “hourglass” shape, where the 2 ends of the THV expand more than the mid-section (i.e., the narrowest level exists between the leaflet inflow and leaflet outflow and usually corresponds to the neo-annulus/sewing ring of the SHV) and a “cone” shape, where the expansion area gradually increases from the inflow to the outflow. MViV: mitral valve-in-valve
IN VIVO ASSESSMENT FOR THV DEFORMATION AFTER MVIV IMPLANTATION
Analyses of THV configuration after implantation were performed in a single phase between mid- to end-systole (20 to 40% of the R-R interval), where the best image quality without motion artefact was obtained.
1. Oversizing: oversizing was calculated based on the size of THV and the True ID of SHV as:
Oversizing (mm)=Size of THV–True ID of SHV
2. Implant depth: implant depth was measured as the average of the maximum and minimum distances between the inflow aspect of THV and SHV, i.e., atrial projection of the THV above the sewing ring of the SHV (Figure 2).
3. Canting: canting was measured as the angle between the axes of the THV and SHV (Figure 2).
4. Deformation of the THV: the analysis of THV deformation comprised the degree of stent frame expansion, eccentricity, neosinus volume index and asymmetric leaflet expansion; this was performed as described in detail previously11 and as summarised in Supplementary Table 1
4.1. THV configuration: The design intent of the S3/S3 Ultra prosthesis is a cylindrical shape with the same cross-sectional area throughout the frame. However, due to the features of the MViV procedure, i.e., oversizing and desirable flaring at the outflow to prevent embolisation, a cylindrical shape may not be achievable. Hence, the THV configuration after implantation was studied comparing the in vitro and in vivo configurations.
4.2. THV deformation index: The prosthesis deformation index was calculated as follows:
THV deformation index=(area at leaflet outflow+area at leaflet inflow)/(2×area at the narrowest level)
When the THV expands to be perfectly cylindrical, this index would be 1.00, whereas an index of >1 represents varying degrees of THV deformation.
4.3. Vertical deformation: The minimum and maximum heights of the THV were measured, and vertical deformation was calculated as the difference between the two measurements in mm (Figure 2). The relative location of the minimum height of the THV to the mitral valve annulus was identified using multiplanar reconstruction.
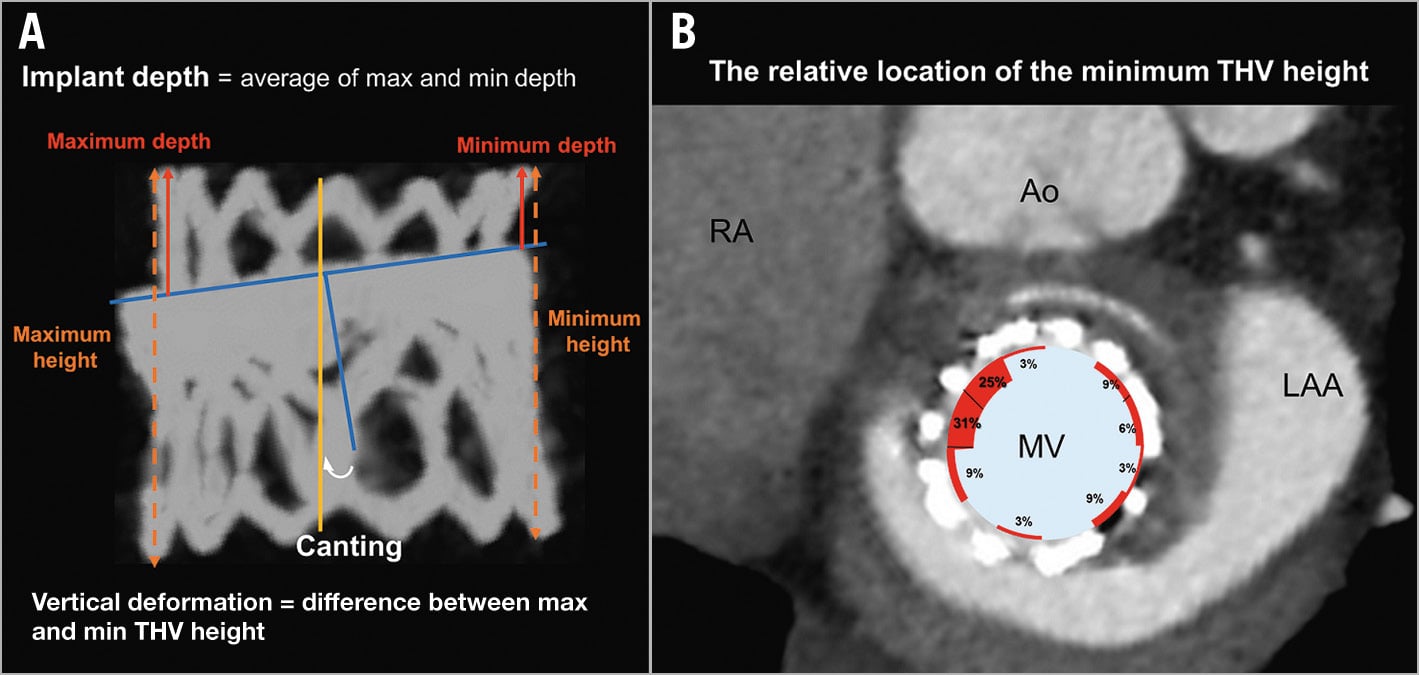
Figure 2. Implant depth, canting and vertical deformation. A) To calculate the implant depth of the transcatheter heart valve (THV), the maximum and minimum depths from the inflow aspect of the surgical heart valve (SHV) to that of the THV are measured and averaged (red arrows). Canting is measured as the angle between the axes of the THV and the SHV. For vertical deformation of the THV, the maximum and minimum heights of the THV (orange dotted lines) were measured and we calculated the difference between the THV heights. B) The relative location of the minimum height of the transcatheter heart valve to the MV annulus was identified using multiplanar reconstruction. The figure is from the left ventricle view. Ao: aortic valve; LAA: left atrium appendage; max: maximum; min: minimum; MV: mitral valve; RA: right atrium.
HALT ASSESSMENT
HALT is visually identified increased thickness of the THV leaflet on the in vivo CT according to Valve Academic Research Consortium (VARC)-3 criteria. Using multiplanar reformats, each prosthetic leaflet was evaluated for the presence of HALT in at least 2 different diastolic phases, and the severity of HALT was semiquantitatively assessed on long-axis views, carefully aligned with the leaflet centre regarding involvement along the curvilinear leaflet beginning at the base, using a 5-tier grading scale: none, ≤25%, 26%-50%, 51%-75%, and >75%17.
ECHOCARDIOGRAPHIC ASSESSMENT
A non-invasive assessment of THV haemodynamics was performed using transthoracic echocardiography 30 days after MViV with standard assessments using several systems (Philips iE33, EpiQ, CX50; Philips Medical Systems). The mean gradient (MG) across the THV was measured with the modified Bernoulli equation.
STATISTICAL ANALYSIS
The electronic medical records were reviewed for collection of clinical characteristics, comorbidities, procedural characteristics, and clinical outcomes. Categorical and continuous variables were expressed as frequencies (percentages) and medians (interquartile ranges [IQR]), respectively, and compared using the Kruskal-Wallis test. Due to the small cohort size, variables associated with THV deformation or haemodynamic status after the procedure were assessed using univariable linear regression analysis but not by multivariable model analysis. Correlations were examined with a calculation of Pearson coefficients to assess the relationship between the degree of oversizing and the THV deformation index. P-values<0.05 were considered a priori statistically significant. All analyses were performed using SPSS version 25 (IBM).
Results
IN VITRO MEASUREMENTS OF SAPIEN 3
The median external stent frame areas for SAPIEN 3 sizes 23, 26 and 29 mm were 401 (IQR 400-402) mm2, 521 (IQR 520-522) mm2 and 650 (IQR 650-650) mm2, respectively. The neosinus volumes for valve sizes 23, 26 and 29 mm were 3.71 ml, 5.97 ml and 8.00 ml, respectively.
STUDY COHORT
Overall, 38 patients with SHV degeneration underwent MViV implantation with a balloon-expandable SAPIEN 3 or 3 Ultra device during the study period. Those without a postprocedural CT examination (n=5) were excluded, leaving a final study cohort of 33 patients. The post-procedure CT was performed at a median of 35 (IQR 31-47) days after MViV implantation, and adequate image quality for THV assessment was present in all 33 patients. Patients were predominantly elderly (median age: 81 [IQR 76-86] years), 69.7% were on anticoagulation at baseline and had high surgical risk as assessed by the Heart Team (Society of Thoracic Surgeons Predicted Risk of Mortality score: 7.1% [IQR 3.6-11.9%]) (Table 1). Mitral regurgitation was the predominant pathology of SHV degeneration, present in 18 (54.5%) patients. The nominal balloon inflation volume was used during the procedure in all but three patients in whom the S3 size used was either equal to or 0.5 mm larger than the True ID (i.e., 0 or 0.5 mm oversize). In these cases, an extra volume (1 or 2 cc) was added. Post-implant balloon dilatation was performed during the procedure in 3 patients (9.1%) using a True Balloon (Midwest Interventional Systems).
Table 1. Clinical and procedural characteristics (N=33).
| Baseline clinical characteristics | |
|---|---|
| Age, years | 81 [76-86] |
| Male | 14 (42.4) |
| Duration between SMVR and mitral ViV, years | 10 [7-12] |
| Diabetes mellitus | 6 (18.2) |
| Hypertension | 24 (72.7) |
| Atrial fibrillation/flutter | 22 (66.7) |
| Coronary artery disease | 13 (39.4) |
| eGFR, ml/min/1.73 m2 | 48 [42-69] |
| Anticoagulant therapy | 23 (69.7) |
| STS-PROM score, % | 7.1 [3.6-11.9] |
| Baseline echocardiographic characteristics | |
| Reason for SHV dysfunction | |
| Mitral regurgitation | 18 (54.5) |
| Mitral stenosis | 8 (24.2) |
| Mixed mitral stenosis and regurgitation | 7 (21.2) |
| LV ejection fraction, % | 60 [56-65] |
| LV stroke volume index, ml/m2 | 26 [22-36] |
| MVG, mmHg | 10 [7-13] |
| Prosthesis valve characteristics | |
| SHV leaflet/stent frame type | |
| Porcine/polymer | 8 (24.2) |
| Pericardial/metal+polymer | 25 (75.8) |
| SHV True ID | |
| 20.5/21/22 mm | 4 (12.1) |
| 23/24/25 mm | 11 (33.3) |
| 26/27/28/28.5 mm | 18 (54.5) |
| THV size | |
| #23 | 1 (3.0) |
| #26 | 10 (30.3) |
| #29 | 22 (66.7) |
| Valve-in-valve procedural characteristics | |
| Transseptal access to mitral valve | 30 (90.9) |
| Balloon-volume filling | |
| Nominal filling | 30 (90.9) |
| Overfilling | 3 (9.1) |
| Post-balloon dilatation | 3 (9.1) |
| Medications at discharge | |
| Antiplatelet therapy | 29 (87.9) |
| Anticoagulant therapy (warfarin) | 32 (97.0) |
| At 1-month follow-up | |
| PT-INR (n=32) | 2.2 [1.8-2.5] |
| ≥2.5 | 9 (28.1) |
| 2.0-2.4 | 13 (40.6) |
| <2.0 | 10 (31.3) |
| MVG, mmHg (on echocardiography) | 9 [6-11] |
| Values are median [interquartile range] or n (%). eGFR: estimated glomerular filtration rate; ID: internal diameter; LV: left ventricular; MVG: mitral valve gradient; PT-INR: prothrombin time and international normalised ratio; SHV: surgical heart valve; SMVR: surgical mitral valve replacement; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve; ViV: valve-in-valve | |
OVERSIZING, IMPLANT DEPTH AND CANTING
Oversizing THV with respect to the True ID of the SHV prosthesis was common, with a median value of 2.0 (1.0-3.5) mm (Table 2). The oversizing used was ≤1, 2, 2.5-3, and ≥4 mm in 10, 8, 7 and 8 patients, respectively. In 7 patients with borderline True ID of SHV (i.e., True ID of SHV=22 or 25 mm), a larger size of THV (i.e., 4 mm oversize) was chosen in 6 patients and a smaller one (i.e., 1 mm oversize) in 1 patient. An extreme oversize of 5 mm was used in 2 patients, only during the early experience, and was most likely due to use of the stent ID rather than True ID for sizing of the THV (Supplementary Table 2). The median implant depth (i.e., larger % of the THV towards the left atrium) was 6.2 (3.8-7.8) mm. Minimal or no canting (i.e., <10º) occurred in 30 (90.9%) patients, while 3 had canting >10º.
Table 2. One-month postprocedural cardiac computed tomography variables (N=33).
| LV ejection fraction, % | 48 [41-52] |
| LV stroke volume index, ml/m2 | 36 [30-41] |
| Relationship between THV and SHV | |
| Oversizing, mm | 2.0 [1.0-3.5] |
| Implant depth, mm | 6.2 [3.8-7.8] |
| Canting, degree | 4.7 [3.1-8.1] |
| THV deformation | |
| Expansion, % | |
| Leaflet inflow (LA side) | 82.3 [75.4-88.8] |
| Narrowest level | 74.0 [68.1-84.1] |
| Leaflet outflow (LV side) | 99.8 [92.4-103.8] |
| Eccentricity | |
| Leaflet inflow (LA side) | 0.25 [0.17-0.31] |
| Narrowest level | 0.24 [0.17-0.33] |
| Leaflet outflow (LV side) | 0.28 [0.20-0.33] |
| THV deformation index | 1.21 [1.13-1.29] |
| THV configuration | |
| Hourglass shape | 30 (90.9) |
| Cone shape | 3 (9.1) |
| Vertical deformation, mm | 1.7 [0.9-2.4] |
| Neosinus volume index | 0.82 [0.78-0.88] |
| Asymmetric leaflet expansion, degree | 12 [8-19] |
| Values are median [interquartile range] or n (%). LA: left atrium; LV: left ventricular; SHV: surgical heart valve; THV: transcatheter heart valve | |
THV DEFORMATION
Relative to the nominal area of the prosthesis in vitro, underexpansion of the implanted THV was observed at all levels of the S3/S3 Ultra frame following the MViV procedure (Table 2), but circularity was maintained, with eccentricity ranging from 0.24 to 0.28, across all levels of the THV, suggesting a near-circular underexpansion throughout the THV.
Overall, THV stent frame deformation was common, with a median THV deformation index of 1.21 (IQR 1.13-1.29). Two distinct configurations of the S3/S3 Ultra frame were observed after MViV implantation: an “hourglass” shape, where the 2 ends of the THV expand more than the mid-section (i.e., the narrowest level exists between the leaflet inflow and leaflet outflow and usually corresponds to the neo-annulus/sewing ring of the SHV), and a “cone” shape, where the expansion area gradually increases from the inflow to the outflow. An hourglass configuration was common and was observed in 30 (90.9%) patients, whereas a cone shape was observed in only 3 (9.1%) patients (Figure 1). Vertical deformation, i.e., difference in THV height of ≥2 mm between the maximum and minimum, was observed in 11 (33.3%) patients. The minimum THV height more often occurred medially (near the interatrial septum) than laterally (near the left atrial appendage) (Figure 2).
The neosinus volume was smaller than the nominal volume in vitro in all but one patient. The range of neosinus volumes observed was 31-50% smaller in 3 patients, 10-30% in 25 and 0-9% in 4, compared to the nominal volume. In one patient where the neosinus volume was slightly greater than nominal (neosinus volume index, 1.03), the oversize was 0.5 mm for a 29 mm SAPIEN 3 prosthesis, and the THV deformation index was 1.09. In the majority of patients, some degree of leaflet asymmetry was observed with <10º, 10º to 20º, and 21º to 30º in 9, 18 and 6 patients, respectively.
DETERMINANTS OF THV DEFORMATION
The degree of oversizing and final configuration of the THV within the SHV affected the extent of the THV deformation (Table 3). A greater degree of oversizing was associated with a greater degree of underexpansion at the narrowest level of the THV, with a higher THV deformation index and, consequently, a smaller neosinus volume index (Figure 3, Supplementary Table 3). A shallower implant depth (i.e., larger % of the THV towards the left atrium) was also related to a greater degree of underexpansion and a smaller neosinus volume index. Canting was associated with worse eccentricity and greater vertical deformation.
Table 3. Determinants of THV deformation.
| Expansion at the narrowest area | Eccentricity at the narrowest area | THVdeformation index | Vertical deformation | Neosinus volume index | Asymmetric leaflet expansion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | |
| Oversizing, mm | –0.634 | <0.001 | –0.167 | 0.35 | 0.594 | <0.001 | 0.071 | 0.69 | –0.588 | <0.001 | 0.158 | 0.38 |
| Implant depth, mm | –0.413 | 0.02 | –0.002 | 0.99 | 0.255 | 0.15 | 0.289 | 0.10 | –0.425 | 0.01 | 0.241 | 0.18 |
| Canting, degree | 0.208 | 0.25 | 0.480 | 0.005 | –0.370 | 0.03 | 0.411 | 0.02 | –0.109 | 0.55 | –0.104 | 0.57 |
| ≥Moderate MS due to SHV degeneration | 0.286 | 0.11 | 0.224 | 0.21 | –0.430 | 0.01 | –0.097 | 0.59 | –0.135 | 0.45 | –0.094 | 0.60 |
| Univariable linear regression analysis was performed for each THV deformation. MS: mitral stenosis; SHV: surgical heart valve; THV: transcatheter heart valve | ||||||||||||
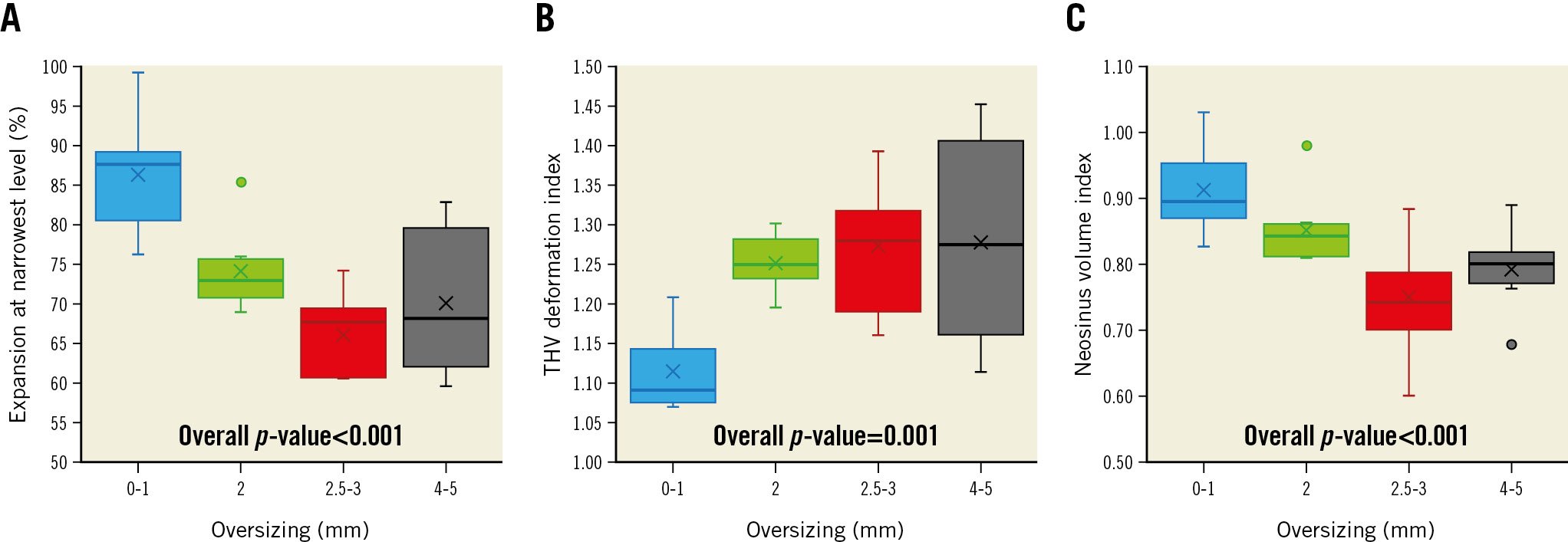
Figure 3. Oversizing and transcatheter heart valve deformation. As oversizing increases, the expansion area at the narrowest level of the stent frame decreases (A), THV deformation index increases (B) and neosinus volume index decreases (C). The horizontal lines within the boxes correspond to the median. The bottom of the boxes indicates the 25th percentile (quartile 1), and the top of the boxes represents the 75th percentile (quartile 3). The T-bars that extend from the boxes represent scores outside the middle 50% and the lowest and highest scores. The Xs in the boxes correspond to the mean, and the dots outside of the boxes and T-bars represent outliers. THV: transcatheter heart valve
HALT AND HAEMODYNAMICS
Overall, the incidence of HALT on the 30-day postprocedural CT was 27.3% (9 of 33 patients), of which all patients except one had HALT on multiple leaflets or >26-50% HALT on at least one leaflet (Figure 4). Most patients (32 of 33) were on anticoagulation therapy with warfarin except one due to contraindication. However, the prothrombin time and INR (PT-INR) at the time of the CT scan was below the target range (i.e., <2.5) in 23 of 32 patients (Table 1). Only 2 of 9 patients with HALT had a PT-INR within the therapeutic range (i.e., ≥2.5). Notably, among patients with a PT-INR of <2.5, HALT was predominantly observed with a high THV deformation index of ≥1.18, while no HALT was observed with a THV deformation index of <1.18 (Central illustration).
The mean mitral valve gradient (MVG) on the 30-day postprocedural echocardiography was 9 (6-11) mmHg. This tended to be associated with the type and size of the SHV, oversizing and the degree of THV expansion at the leaflet inflow but was not associated with the presence of HALT (Table 4).
Of the 9 patients with HALT, 4 patients had a follow-up CT 3 months later, 3 of whom continued to demonstrate HALT despite being within the therapeutic range of anticoagulation, while one had resolution of HALT. The remaining 5 patients did not have follow-up CTs due to social reasons. All 9 remain on lifelong anticoagulation.
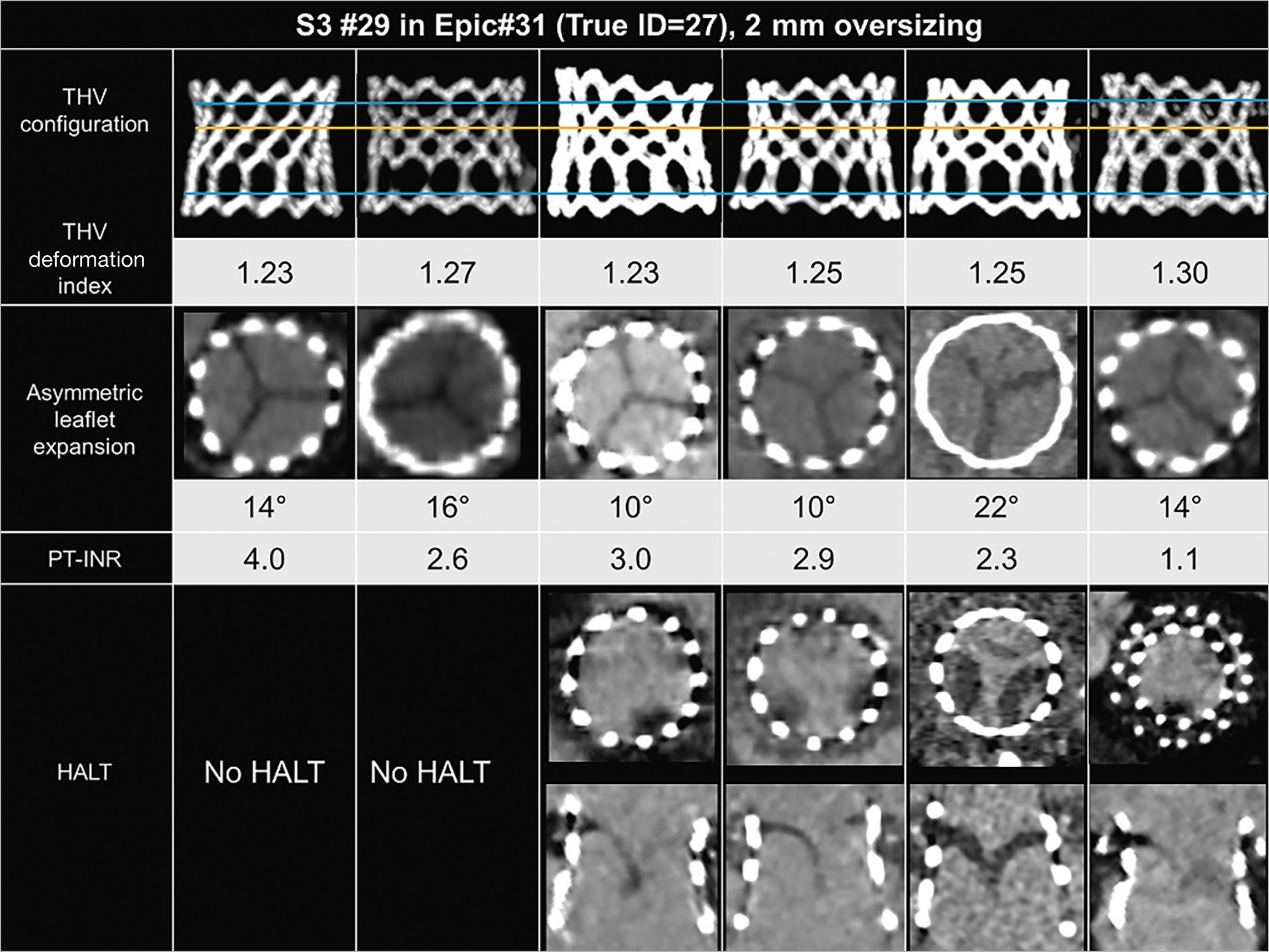
Figure 4. Transcatheter heart valve deformation after MViV implantation. Example cases of 29 mm SAPIEN 3 (S3) in Epic #31 SHV (True ID=27 mm) with and without hypoattenuating leaflet thickening (HALT) on 30-day postprocedural computed tomography. Yellow lines correspond to the narrowest level of THV, and blue lines correspond to the leaflet inflow and leaflet outflow levels. ID: internal diameter; MViV: mitral valve-in-valve; PT-INR prothrombin time and international normalised ratio; SHV: surgical heart valve; THV: transcatheter heart valve
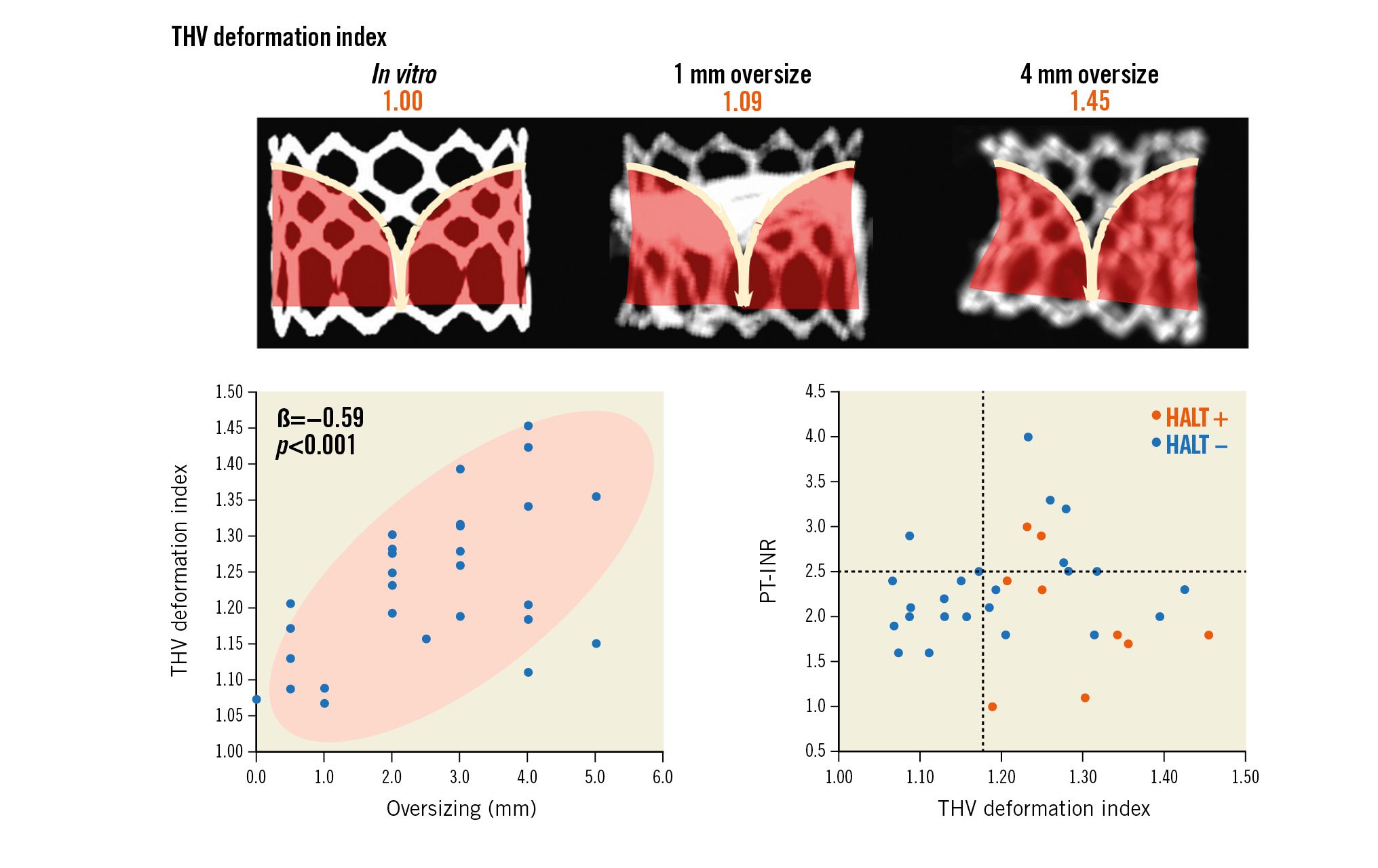
Central illustration. Stent frame deformation of transcatheter heart valves (THV), i.e., underexpansion and an hourglass shape, is common and is influenced by the degree of oversizing. Among patients with a prothrombin time international normalised ratio (PT-INR) of <2.5, hypoattenuating leaflet thickening (HALT) was predominantly observed when the THV deformation index was high, i.e., ≥1.18, while no HALT was observed when the THV deformation index <1.18.
Table 4. Determinants of postprocedural MVG at 30 days.
| Standardised β | p-value | |
|---|---|---|
| Age, years | 0.168 | 0.35 |
| Male | −0.294 | 0.10 |
| Body mass index, kg/m² | 0.032 | 0.88 |
| ≥moderate MS due to SHV degeneration | 0.138 | 0.45 |
| Porcine/polymer SHV (vs pericardial/polymer+metal) | 0.321 | 0.07 |
| SHV True ID ≤22 mm | 0.330 | 0.06 |
| THV #29 (vs #23 or #26) | −0.211 | 0.24 |
| Post-balloon dilatation | 0.051 | 0.78 |
| Postprocedural CT-derived LV ejection fraction, % | 0.149 | 0.41 |
| Postprocedural CT-derived LV stroke volume index, ml/m² | −0.148 | 0.41 |
| Oversizing, mm | 0.310 | 0.08 |
| Implant depth, mm | 0.040 | 0.83 |
| Canting, degree | −0.146 | 0.42 |
| Expansion at leaflet inflow (LA side), % | −0.328 | 0.06 |
| Expansion at narrowest level, % | −0.199 | 0.27 |
| Expansion at leaflet outflow (LV side), % | 0.053 | 0.77 |
| Eccentricity at narrowest level | −0.232 | 0.19 |
| THV deformation index | 0.121 | 0.50 |
| Difference of THV height, mm | −0.081 | 0.65 |
| Neosinus volume index | −0.177 | 0.33 |
| Asymmetric leaflet expansion, degree | −0.376 | 0.03 |
| HALT | −0.098 | 0.59 |
| CT: computed tomography; HALT: hypoattenuating leaflet thickening; ID: internal diameter; LA: left atrium; LV: left ventricular; MS: mitral stenosis; MVG: mitral valve gradient; SHV: surgical heart valve; THV: transcatheter heart valve | ||
Discussion
The present investigation is the first study to systematically evaluate the extent and predictors of THV deformation following MViV with a balloon-expandable SAPIEN device. The key findings are as follows: 1) THV deformation, i.e., underexpansion and an hourglass shape, is common after implantation; 2) such THV deformation is negatively affected by an excessive oversizing and a shallower implant depth (i.e., larger % of the THV towards the left atrium), and 3) there is a higher incidence of HALT in patients with a higher degree of deformation and an INR <2.5. Taken together, these findings might have implications in optimising MViV procedures and achieving better mid- and long-term clinical outcomes.
Prior reports have shown the safety and effectiveness of MViV therapy for patients with SHV degeneration1234. The number of patients who need this therapy is expected to grow with the increasing use of bioprosthetic surgical valves and approval of MViV as an indication for high-risk patients18. Nevertheless, the long-term durability of THV with MViV remains uncertain. Recent findings on TAVR, ViV and in vitro models have shown that the deformation of THV prostheses might increase the risk of THV dysfunction with the potential consequences of pinwheeling, leaflet shear stress and increased flow stagnation leading to HALT78910111920212223. In addition, there is higher risk of valve thrombosis in the mitral position, as evident from surgical and transcatheter mitral valve replacement experience24. Hence, minimising THV deformation after an MViV procedure may be important for the long-term durability of THV.
Compared to the results of our previous study of TAVR in native aortic stenosis11, we observed that the SAPIEN prosthesis after MViV implantation was more underexpanded (94% vs 82% at the leaflet inflow; 89% vs 74% at the waist level) and more deformed (THV deformation index: 1.09 vs 1.21). In this study, excessive oversizing and a shallower implant depth (i.e., larger % of the THV towards the left atrium) were factors associated with a greater degree of underexpansion and THV deformation index.
As embolisation is a serious complication after MViV procedures, oversizing of at least 2 mm and the adoption of a flared or conical shape for THV after implantation are recommended due to early experience of embolisation with the SAPIEN XT6. However, this contributes to deformation, especially in borderline sizes. Newer-generation devices like the SAPIEN 3/3 Ultra have a different stent structure, with a higher stent height and an outer skirt, which offers an opportunity to reduce THV deformation by minimising oversizing. As SAPIEN devices are only available in 3 mm increments, the considerations above become relevant in patients with borderline SHV sizes where the current available sizes of the SAPIEN device could result in either 1 or 4 mm oversizing (Supplementary Table 4). Bioprosthetic valve fracture (BVF) is utilised increasingly during ViV procedures and can be also considered to optimise expansion during MViV procedures2526. When excessive oversizing is unavoidable and/or significant THV deformation is observed on the operating table, BVF could be considered to optimise THV expansion after weighing the procedural risks, as it could offer superior THV expansion with lower residual transvalvular gradients2627. Vertical deformation was observed in 33.3% of patients and partly reflects the issues with the transseptal approach and orientation of the SHV in the mitral position as well as left ventricular cavity shape. Techniques to optimise trajectory can minimise this issue and also allow the operator to implant the valve in a shallow position, as higher implantations in the atrium were associated with more deformation. Further studies are needed.
Although HALT was observed in 27.3% of patients (9 of 33) on the 30-day postprocedural CT in this investigation, 24 of 33 patients were on insufficient anticoagulation therapy (i.e., 1 patient with no anticoagulation therapy and 23 with PT-INR <2.5). However, 2 of 9 patients with therapeutic PT-INR demonstrated HALT. This highlights the importance of adequate anticoagulation and routine follow-up, as the risk of HALT may be higher in the mitral position, with patient-related risk factors such as atrial fibrillation and left ventricular dysfunction24.
Based on the results of prior studies891011 and the present study, excessive THV deformation might be associated with an increased risk of HALT and, subsequently, concern about THV durability, but further studies in larger number of patients with sufficient anticoagulation therapy will be needed to understand factors contributing to the occurrence of HALT after MViV implantation. THV deformation and final configuration may be more important in the mitral position, as four of our patients with similar MViV combinations, i.e., 31 Epic SHV (Abbott) and 29 SAPIEN 3 (oversize of 2 mm), demonstrated varying degrees of HALT but also different final configurations (Figure 4).
Similar to what is seen with TAVR in native aortic stenosis, neither the THV deformation index nor the presence of HALT with MViV procedures were associated with an increase in MVG, suggesting it is subclinical and often unable to be detected by transthoracic echocardiography. This explains the lower incidence of clinical thrombosis (2.4%) after MViV therapy reported previously when assessed by transthoracic echocardiography14. Based on these findings, cardiac CT after MViV implantation might be considered in the short-term follow-up of these patients, as persistent HALT, if untreated, could affect THV degeneration via a process of leaflet fibrosis and then calcification2628.
Limitations
First, although this is the first study to comprehensively assess postprocedural CT, which was prospectively performed 30 days after MViV implantation, the number of patients is small, and the analysis is limited especially for factors associated with HALT. Second, despite our institutional anticoagulation protocol following MViV procedures, more than half of the patients were on insufficient anticoagulation therapy at the time of the CT scan, highlighting the real-world issues. Third, we did not assess pinwheeling of THV prosthesis leaflets; its assessment by CT remains challenging and can only be performed when excellent image quality is obtained throughout the entire diastolic phase. Lastly, the long-term effects of untreated HALT cannot be discerned from our study. As our protocol does not have longitudinal CT follow-up more than 30 days after the procedure if no HALT observed, our data cannot be used to evaluate later occurrences of HALT in these patients.
Conclusions
THV deformation, i.e., underexpansion and an hourglass shape, is common after MViV implantation with the currently available SAPIEN 3 sizes, and its key determinant is excessive oversizing. The relationship between deformation and HALT needs further study, although those with a higher deformation index and lower INR appeared to be at most risk. Optimisation of THV expansion and a higher and consistent INR target, i.e., >2.5, may be required to prevent HALT following MViV procedures.
Impact on daily practice
The deformation of transcatheter heart valves (THV), i.e., underexpansion and an hourglass shape, is common after mitral valve-in-valve implantation with a SAPIEN 3 prosthesis, and its key determinant is excessive oversizing and a shallower implant depth (i.e., larger % of the THV towards the left atrium). Since excessive THV deformation can cause THV dysfunction, avoiding excessive oversizing and a shallower implant depth and using post-dilatation should be considered to optimise THV expansion.
Conflict of interest statement
J.L. Cavalcante has received consulting fees from 4C Medical, Abbott Structural, Anteris, Aria CV, Boston Scientific, Edwards Lifesciences, HighLife, Medtronic, VDyne, W.L. Gore & Associates, and Xylocor; and has received research grant support from Abbott Northwestern Hospital Foundation. P. Sorajja has received consulting fees from 4C Medical, Abbott Structural, Anteris, Boston Scientific, Edwards Lifesciences, Evolution Medical, Foldax, GLG, Medtronic, Philips, Siemens, Shifamed, W.L. Gore & Associates, VDyne, and xDot Medical; and has received institutional research grant support from Abbott Structural, Medtronic, and Boston Scientific. M. Enriquez-Sarano has received consulting fees from CryoLife, Edwards Lifesciences, HighLife, and ChemImage. V.N. Bapat has received consulting fees from Abbott Structural, Medtronic, Boston Scientific, Edwards Lifesciences, 4C Medical, and Anteris. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

