
Abstract
Aims: This study aimed to assess the potential relationship between subclinical leaflet thickening and stent frame geometry in patients who underwent aortic valve replacement with a self-expanding transcatheter heart valve (THV).
Methods and results: Seventy-five patients with a self-expanding THV were studied with 4D-computed tomography and analysed for leaflet thickening. There was no difference in THV size, overall THV expansion, eccentricity or implantation depth between patients with and those without leaflet thickening. Moderate-to-severe regional THV underexpansion (≤90°) more frequently occurred at the non-coronary and right coronary cusps with a significantly higher incidence of leaflet thickening than in cases of full regional THV expansion (24% vs. 3%, p<0.01). Regional THV underexpansion at the inflow level more often translated into the same issue at the valvular level in THV with intra-annular as compared to supra-annular valve position (54% vs. 17%; p=0.04). In case of post-dilatation, regional THV underexpansion occurred less frequently as compared to THV that were not post-dilated (18% vs. 43%, p=0.028). A similar but non-significant trend was found for leaflet thickening.
Conclusions: Regional THV stent frame underexpansion is associated with an increased risk of leaflet thickening. Post-dilatation of self-expanding THV as well as a supra-annular valve position seem to reduce the occurrence of this phenomenon.
Abbreviations
AS: aortic valve stenosis
4DCT: four-dimensional computed tomography
HALT: hypoattenuating leaflet thickening
HAM: hypoattenuation affecting motion
LCC: left coronary cusp
NCC: non-coronary cusp
NOAC: novel oral anticoagulation
OAC: oral anticoagulation
RCC: right coronary cusp
TAVR: transcatheter aortic valve replacement
THV: transcatheter heart valve
Introduction
Transcatheter aortic valve replacement (TAVR) has become an established therapeutic option for patients with symptomatic, severe aortic valve stenosis (AS) who are ineligible or at high risk for conventional cardiac surgery1-3. In recent years, TAVR technology has also been increasingly used to treat patients with an intermediate-risk profile. This practice is supported by results from the NOTION and PARTNER II trials indicating that TAVR is a viable option for patients with a lower risk profile1,4.
Recently, it has been reported that subclinical leaflet thickening and abnormal leaflet motion may be found in both transcatheter and surgical bioprosthetic aortic valves5-7. Thrombogenic pathway(s) seem to be involved, since leaflet thickening can resolve with oral anticoagulant (OAC) therapy. The clinical implications of this phenomenon are currently unknown and the responsible mechanisms have not yet been identified8. Interestingly, in vitro studies have recently demonstrated that eccentric transcatheter heart valve (THV) stent distortion was associated with bending of the leaflets and increased commissure strain9. Accordingly, we hypothesised that mechanical factors may be related to the development of these bioprosthetic leaflet abnormalities.
This study aimed to assess the potential relationship between THV stent frame characteristics and leaflet abnormalities based on the analysis of four-dimensional computed tomography (4DCT) scans in patients who had undergone TAVR with self-expanding THV.
Methods
STUDY POPULATION
Patients with severe tricuspid AS who underwent TAVR with a self-expanding CoreValve® (Medtronic, Minneapolis, MN, USA), Evolut™ R (Medtronic) or Portico™ (St. Jude Medical, St. Paul, MN, USA) THV were included in the study. CoreValve is a non-repositionable THV with supra-annular valve position; Evolut R is a repositionable THV with supra-annular valve position, and Portico is a repositionable THV with intra-annular valve position. All TAVR procedures were performed in Denmark or Japan between December 2013 and July 2016. The patients included in this study were randomly selected; the only exclusion criteria were a glomerular filtration rate (GFR) <45 mL/min and TAVR performed less than three weeks or more than one year ago (median 97 days; range 24-331 days). All Danish patients are part of the SAVORY registry (NCT02426307). In accordance with the institution’s policies, all patients gave written informed consent for TAVR and the use of anonymous data for research in accordance with the EC review board approval (RH-2016-111, Suite# 04625).
COMPUTED TOMOGRAPHY ACQUISITION
Image acquisition was performed using latest-generation scanners, Aquilion ONE™ ViSION Edition (Toshiba Medical Systems, Tochigi, Japan) and Brilliance iCT (Philips Medical Systems, Best, the Netherlands) with a retrospective ECG-gating scan protocol. An image noise standard deviation level of 30 was predefined, which resulted in a variable kV and mA between patients. The kV was set to 120 by default; if this resulted in a maximal mA, the kV was raised, until a submaximal mA was reached. To minimise radiation exposure, a dose modulation approach was used reducing dose in the 55-100% RR range (diastole). A cardioselective beta-blocker (metoprolol 25-150 mg) was administered orally approximately one hour before scanning in patients with a heart rate >60 bpm and no contraindications for this drug. In patients with metoprolol contraindications (allergic asthma or left ventricular ejection fraction <30%), a single dose of oral ivabradine (15 mg) was given two hours prior to the CT scan. An intravenous line (18 gauge) was inserted in an antecubital vein. Intravenous contrast media (Visipaque™; GE Healthcare, Chalfont St. Giles, UK) was infused with a flow rate of 5 ml/s using a triphasic injection protocol (60-100 ml, 320 mg/ml depending on weight). Image acquisition triggering was set at a left ventricle attenuation density of 180 Hounsfield units. Raw data of the contrast scan were reconstructed in 5% intervals throughout the cardiac cycle with a slice thickness and increment of 0.5/0.5 mm allowing image analysis in both systole and diastole. A kernel FC3 back projection filter and AIDR 3D (Toshiba Medical Systems) was used.
COMPUTED TOMOGRAPHY ANALYSIS
LEAFLET ABNORMALITIES
Bioprosthesis leaflet thickening and motion were assessed by 4DCT scan as previously described6. The status of each prosthetic leaflet was deemed either normal or abnormal with respect to morphology and motion. Hypoattenuating leaflet thickening (HALT) was defined as a focal hypoattenuating abnormality attached to the prosthetic leaflet, or diffuse thickening of a prosthetic valve leaflet detectable in at least two orthogonal reconstructed planes. Leaflet thickening affecting motion – referred to as hypoattenuation affecting motion (HAM) – was defined as more than 50% reduced systolic leaflet excursion or immobilisation of a leaflet with HALT.
THV STENT FRAME ANALYSIS
This analysis was performed in the end-diastolic phase of the heart cycle. Three levels of the stent frame were defined: the inflow level, the valvular level, and the coaptation level of the THV stent frame. Overall THV stent frame geometry was analysed at the inflow level, whereas the regional THV stent frame geometry was analysed at both the inflow level and valvular level. The three prosthesis leaflets were identified at the leaflet coaptation level and 1/3 of the stent struts were allocated to each of the three leaflets (Figure 1).
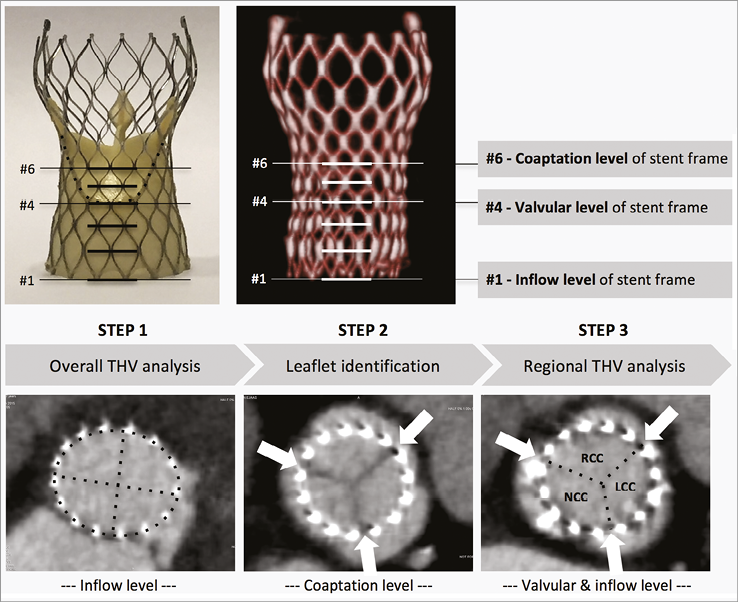
Figure 1. Step-by-step procedure for assessment of THV stent frame geometry. Step 1. Overall THV analysis demands measurement of the minimal and maximal stent frame diameters as well as the stent frame perimeter at the inflow level of the stent frame. Step 2. Identification of the three leaflets at the coaptation level, i.e., where the leaflets meet in the middle of the aorta in a triangular star formation. Arrows are placed to indicate borders between leaflets; one third of the stent struts are allocated to each of the three leaflets. In all levels towards the base of the stent frame, arrows are placed correspondingly. Step 3. The regional THV expansion is evaluated for every single prosthetic leaflet at both the inflow and valvular level by measuring the angle (°) formed by the border stent struts assigned to each prosthetic leaflet and the THV centre point. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp; THV: transcatheter heart valve
OVERALL THV STENT FRAME ANALYSIS
Maximal and minimal stent diameters (mm) and cross-section stent perimeter (mm) were measured at the inflow level of the frame. THV expansion (%) was calculated as (measured THV perimeter/nominal THV perimeter)×100. THV eccentricity (%) was calculated as ([max diameter–min diameter]/max diameter)×1007,10,11. In addition, the stent implantation depth was calculated as the average THV depth below the native aortic annulus, as measured for the three THV leaflets.
REGIONAL THV STENT FRAME ANALYSIS
Regional THV expansion was evaluated by measuring the angle (°) formed by the border stent struts assigned to each prosthetic leaflet and the THV centre point. This angle was measured for all three prosthetic leaflets (non-coronary cusp [NCC], right coronary cusp [RCC], and left coronary cusp [LCC]) at the inflow level and valvular level of the THV stent frame. Full regional THV expansion was defined as an expansion angle >114° (120° with a 5% error margin). Regional THV underexpansion was defined as trivial if angle 102-114°, mild if angle 90-102°, moderate if angle 78-90°, and severe if angle <78°. Intra- and inter-observer agreement for measurement of regional THV stent frame expansion was satisfying (Pearson’s correlation coefficient >0.80); there was 100% agreement on the evaluation of HALT/HAM.
STATISTICAL ANALYSIS
Descriptive statistics were expressed as mean±standard deviation (SD) for continuous variables and as frequency and percentages (%) for discrete variables. The differences in means between groups were determined using the Student’s t-test or Wilcoxon rank-sum test, whereas the chi-square test was used to test for associations between discrete variables. A two-tailed p-value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using commercially available software, SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA).
Results
In this study, 75 patients with symptomatic, severe AS who underwent TAVR with a self-expanding valve were included. Of these, 25 patients were treated with CoreValve, 25 with Evolut R, and 25 with Portico THV. The mean age was 80.9 years and 32% were male. The mean STS risk score was 5.0%. The majority of TAVR procedures (93%) were performed by the transfemoral route. OAC treatment was equally distributed among the three groups (p=0.903). All baseline characteristics are reported in Table 1.
The median radiation dose for acquisition of the 4DCT scan was 11.0 mSv (1.4-30.4 mSv). CT image quality allowed evaluation of HALT/HAM and stent frame expansion in all patients.
LEAFLET ABNORMALITIES
Based on the 4DCT analysis, HALT was found in 13 of the 75 THVs (17%), involving 19 of the 225 leaflets (8%). HALT was detected in two CoreValve (three leaflets), three Evolut R (five leaflets) and eight Portico (11 leaflets) THV (p=0.056). In total, HALT was found in 10/75 (13%) NCC, 7/75 (9%) RCC and 2/75 (3%) LCC (p=0.031).
HAM was found in 11 of 75 THVs (15%), involving 16 of the 225 leaflets (7%). Only three leaflets (two NCC, one LCC) of three different Evolut R THV were found to have HALT without HAM. In total, HAM was found in two CoreValve (three leaflets), one Evolut R (two leaflets), and eight Portico (11 leaflets) THV (p=0.009).
OVERALL THV STENT FRAME ANALYSIS
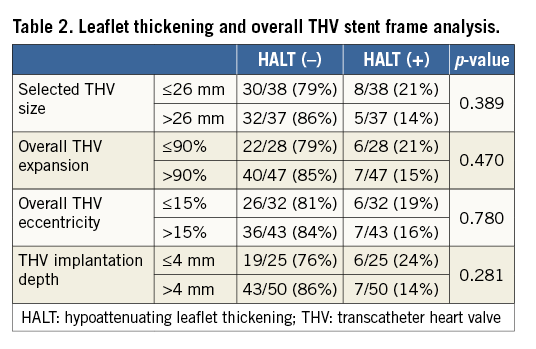
In total, THVs size ≤26 mm were implanted in 38 patients, whereas larger THVs >26 mm were used in 37 patients, with no differences between THV size in the rate of HALT and HAM. As evaluated on 4DCT scan, 47 THV (63%) reached an overall THV expansion >90%, 43 THV (57%) had an eccentricity index >15%, and 50 THV (67%) were measured to have an implantation depth >4 mm. There was no significant difference in overall THV expansion, eccentricity and implantation depth among the different THVs (data not shown), and none of these overall THV stent frame parameters was associated with HALT (Table 2) or HAM (data not shown).
REGIONAL THV STENT FRAME ANALYSIS
The regional THV stent frame expansion was evaluated at the inflow and valvular levels of the stent frame, as illustrated in Figure 2. In CoreValve, the THV stent frame had regional underexpansion at 26/75 (35%) leaflet sites – four of which had moderate-to-severe underexpansion (≤90°) – at the inflow level, translating into moderate-to-severe regional THV underexpansion at one leaflet site at the valvular level (Figure 3). In Evolut R, regional THV underexpansion was observed at 28/75 (37%) leaflet sites – eight of which had moderate-to-severe underexpansion – at inflow level, translating into moderate-to-severe regional THV underexpansion at one leaflet site at the valvular level of the stent frame. In Portico, regional THV underexpansion was present at 23/75 (31%) leaflet sites – 13 of which had moderate-to-severe underexpansion – at the inflow level, translating into moderate-to-severe regional THV underexpansion at seven sites at the valvular level. In total, moderate-to-severe regional THV underexpansion at the inflow level was found at 12/75 (16%) NCC sites, at 13/75 (17%) RCC sites, and at none of the LCC sites (Figure 3, left column).
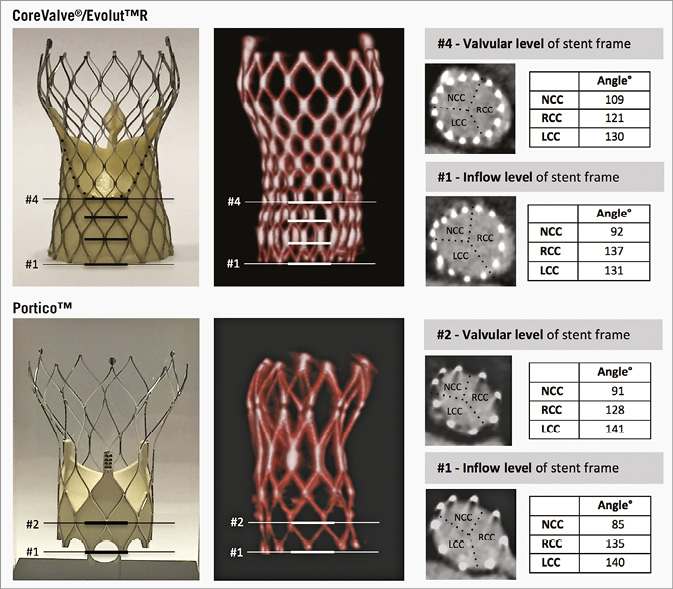
Figure 2. Evaluation of regional THV stent frame expansion. Regional THV expansion (angle, °) was evaluated for every single prosthetic leaflet at both the inflow level and valvular level of the stent frame. CoreValve and Evolut R have a supra-annular valve position, with the valvular level at intersection #4; Portico has an intra-annular valve position with the valvular level at intersection #2. Regional THV underexpansion was defined as trivial if angle 102-114°, mild if angle 90-102°, moderate if angle 78-90°, and severe if angle <78°. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp; THV: transcatheter heart valve
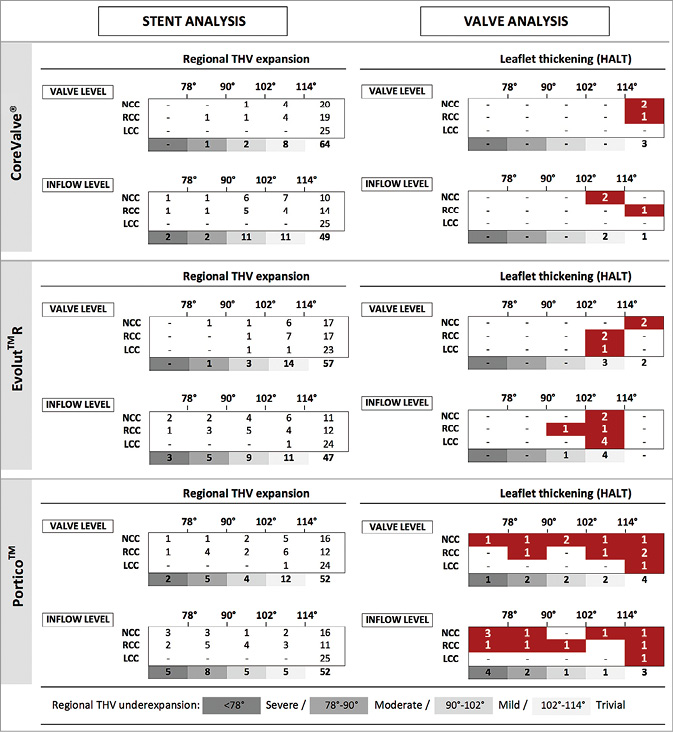
Figure 3. Regional THV expansion and leaflet thickening. The left column shows the degree of regional THV expansion at the inflow and valvular level for the three different self-expanding THVs as evaluated at the NCC, RCC, and LCC sites. The right column shows the number of leaflets with HALT according to the degree of regional THV expansion for the three different THVs analysed. HALT: hypoattenuating leaflet thickening; LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp; THV: transcatheter heart valve
In case of moderate-to-severe regional THV underexpansion (≤90°) at the inflow level, HALT occurred in 6/25 (24%) leaflets vs. in 13/200 (7%) leaflets with mild or less regional THV underexpansion (p=0.008) (Figure 4). A similar result was obtained when analysing for HAM: this phenomenon occurred in 6/25 (24%) leaflets with moderate-to-severe regional THV underexpansion vs. in 10/200 leaflets (5%) with mild or less regional THV underexpansion (p=0.002; data not shown).
Predilatation was performed in 40/75 (53%) of all analysed THV, but moderate-to-severe regional THV underexpansion at the inflow level did not occur less frequently in predilated THV (15/40; 38%) as compared to THV without predilatation (10/35; 29%; p=0.461). Moderate-to-severe regional THV underexpansion more often occurred in THV that were not balloon post-dilated (20/47, 43%) as compared to THV that were post-dilated (5/28, 18%; p=0.028). HALT was observed in 10/47 THV (21.3%) that did not receive post-dilatation vs. in 3/28 THV (10.7%) that were post-dilated (p=0.242).
Discussion
In this study, we assessed the potential association between THV stent frame characteristics and leaflet abnormalities based on the analysis of 4DCT scans in patients who had undergone TAVR with self-expanding THV.
In November 2015, we reported that HALT and HAM are not rare phenomena, occurring in approximately 20% of the studied transcatheter and surgical bioprosthetic aortic valves6. Other groups have confirmed these findings, reporting HALT/HAM rates from 7% to 15% in random TAVR populations12,13. Although the clinical meaning of these findings is still not clear, it has been speculated that leaflet thickening or thrombosis may be related to an increased stroke risk and reduced leaflet durability. Importantly, independent studies have demonstrated that this phenomenon hardly occurs in patients on therapeutic anticoagulation, and that HALT/HAM can – at least temporarily – be reversed by warfarin treatment. Those findings support the hypothesis that the phenomenon is the result of a thrombogenic process.
The reasons why some THVs and/or leaflets would be more prone for this thrombogenic process than others have not been identified. In a recent paper by Hansson et al12, it was stated that a larger THV size might predispose to THV thrombosis, although this finding has not been confirmed by the present study or other data6. In the present study, different self-expanding THV were 4DCT-scanned post TAVR and analysed for overall and regional THV stent frame expansion, eccentricity, and implantation depth. In summary, there was no difference in overall THV stent frame characteristics between patients with and those without leaflet abnormalities. The incidence of HALT and HAM was, however, as high as 25-50% in case of moderate-to-severe regional THV underexpansion, whereas the incidence of this phenomenon was only 3% in case of full regional THV expansion (Figure 4). Only one out of 11 patients (9%) on anticoagulation therapy presented with HALT/HAM (this was one of the seven patients on NOAC treatment), whereas none of the four patients on warfarin treatment had this issue. The reason for the association between regional THV stent frame underexpansion and HALT/HAM is a source of speculation. However, we hypothesise that incomplete stent frame and leaflet expansion may result in “wrinkling” of the leaflet as found in in vitro tests by Gunning et al9, which consequently can make the leaflet more prone to the development of a thin thrombus layer on its surface.
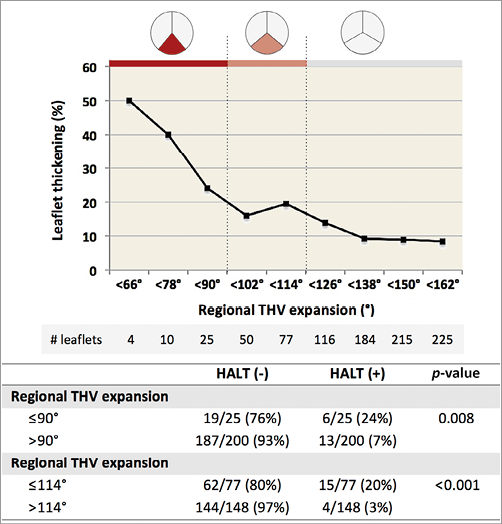
Figure 4. Association of regional THV expansion and leaflet thickening. The graph shows the cumulative incidence of HALT for decreasing degrees of regional THV underexpansion. The panel below shows the HALT incidence rates according to different degrees of regional THV underexpansion – as calculated for regional THV expansion ≤90° and regional THV expansion ≤114°. HALT: hypoattenuating leaflet thickening; HAM: hypoattenuation affecting motion; THV: transcatheter heart valve
The observation that moderate-to-severe regional THV underexpansion at the inflow level of the stent frame is more often seen in Portico than in Evolut R and CoreValve may be explained by at least two factors. Firstly, in order to make new-generation THV resheathable, a trade-off has been a lower radial opening force, which is defined as the force expanding the THV stent frame from a compressed to an expanded state (Figure 5). This radial opening force is less in resheathable THV than non-resheathable THV (Kumar S et al. Stent geometry and radial force comparison of Portico vs CoreValve. Circulation. 2014;130:A16952). The reduced opening force may be a factor resulting in underexpansion due to the fact that constrained stent frames are predominantly found at the non-coronary and right coronary cusps, where initial THV stent frame expansion occurs. Secondly, the Portico has nine stent cells as compared to CoreValve/Evolut R with 15 stent cells (Evolut R 23 mm, which was not included in this study, has 12 stent cells). Fewer stent cells may result in a more pronounced regional underexpansion in case stent struts are caught in the calcium. Also, annulus eccentricity and calcium distribution may have an impact on regional THV underexpansion at the inflow level. However, the sample size of this study is too small to investigate this complex patient-device interaction. Hence, current findings can only be considered hypothesis-generating and will have to be confirmed in future studies.
Another important finding in this study is that moderate-to-severe regional THV underexpansion at the valvular level is very seldom observed in THV with supra-annular valve position (2/150 leaflet sites, 1%) as compared to THV with intra-annular valve position (7/75 leaflet sites, 9%, p<0.001). This observation can most likely be explained by the fact that a constrained frame at the inflow level will gradually resolve towards the outflow part of the stent frame, and thereby be less pronounced at the valvular level for THV with a supra-annular valve position.
Besides THV-specific characteristics, procedure-related factors may also be linked to regional THV underexpansion. Even though predilatation was not associated with the rate of moderate-to-severe regional THV underexpansion at the inflow level of the stent frame, post-dilatation significantly decreases this phenomenon. In other words, if a constrained stent frame is observed following valve deployment – which is best detectable in a right anterior oblique projection (Figure 5) – post-dilatation may be considered.
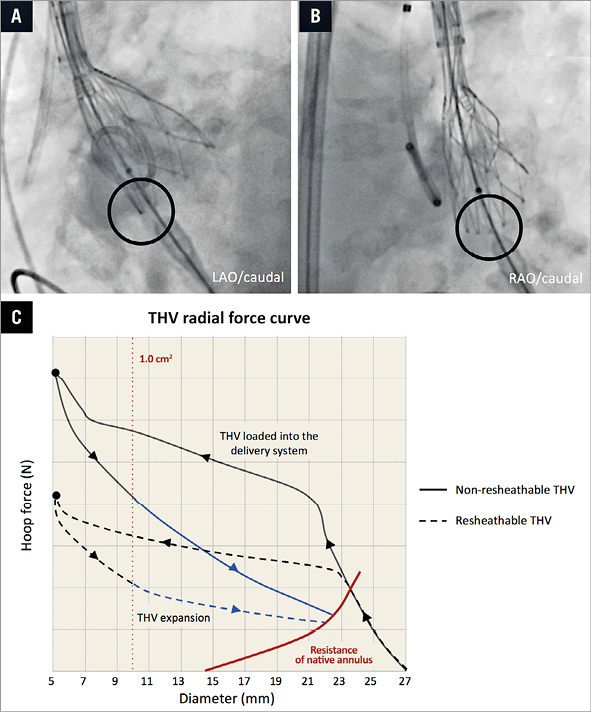
Figure 5. Constrained frame and radial opening force. A) & B) Angiographic images showing a constrained THV stent frame at the NCC site following partial THV deployment, typically best detectable in right anterior oblique (RAO) projection. Since the delivery system is located at the NCC or RCC site during initial THV deployment, regional THV stent frame underexpansion is most likely to occur at these cusps. C) THV radial force curve. The THV prosthesis is loaded and hoop force (N) built up by “crimping” it into the delivery system. During THV deployment into the native aortic annulus, the radial opening force of the THV has to overcome the resistance exerted by the calcified leaflets (blue line) and will most often expand until it meets the outward force of the native annulus (red line). This radial force curve can differ between different types of THV: full line=non-resheathable; dashed line=resheathable. NCC: non-coronary cusp; RCC: right coronary cusp; THV: transcatheter heart valve
In conclusion, the data described in this study indicate that: (1) regional THV underexpansion or a “constrained frame” is associated with an increased risk of HALT/HAM; (2) the number of cells in the THV stent frame may impact on the occurrence of regional underexpansion; (3) THVs with a supra-annular valve position are less prone to have HALT/HAM in case of regional THV underexpansion at the inflow level; and (4) regional THV underexpansion is less prevalent in THVs that were post-dilated during implantation.
Based on these findings, some important conclusions may be drawn. First of all, it is of importance that a constrained frame is detected during the TAVR procedure and a THV post-dilatation is considered in such cases. Secondly, in case of a persistent constrained frame, a closer clinical follow-up with echocardiography or 4DCT may be considered with initiation of OAC therapy in case of increasing transvalvular pressure gradient. In the Copenhagen practice, we typically treat TAVR patients with leaflet thickening with a three-month course of OAC therapy. Only in case of persistent leaflet thickening or moderate-to-severe regional THV underexpansion is a continuation of OAC therapy considered (Figure 6).
Figure 6. Summary figure.
Limitations
Importantly, this study has some limitations that have to be pointed out. The main limitation of this study is the relatively small sample size. However, so far, only very few studies have included more than 50 patients with a post-TAVR 4DCT scan. Moreover, despite very good intra- and inter-observer agreement, angles between stent struts are in some cases difficult to measure; this may induce some degree of analytical error. Another limitation is the variability in the timing of the 4DCT scan post TAVR; this is mainly caused by the retrospective design of the study. As HALT/HAM may be a transient phenomenon, this “timing” variable may have had an impact on the current study findings. Also, the ignorance of patient and annulus characteristics in this study analysis is a weakness and may have introduced some bias. Finally, as the designs of the different THVs differ in more than one way, interpretation of results can be difficult and, hence, some of the conclusions should be considered hypothesis-generating. Larger studies will be needed to investigate this topic in more depth in the future.
Conclusions
This study indicates that regional THV stent frame underexpansion or a “constrained frame” is associated with an increased risk of HALT/HAM. Although results of sub-analyses of this small study cohort should only be considered hypothesis-generating, HALT/HAM seems to occur less frequently in THV with a supra-annular valve position and in case of post-dilatation of these self-expanding valves. Larger-scale, randomised controlled studies are warranted.
| Impact on daily practice Subclinical leaflet thickening has been reported in both transcatheter and surgical bioprosthetic aortic valves. The finding may be related to an increased stroke risk or reduced valve durability. This study indicates that mechanical factors of the transcatheter heart valve (THV) may be related to the development of leaflet thickening. As a result, the findings reported in this study may have an impact on future THV design and on the way transcatheter aortic valve replacement procedures will be performed. |
Conflict of interest statement
O. De Backer and G. Bieliauskas have been consultants for St. Jude Medical. L. Søndergaard has been a consultant and has received research grants from St. Jude Medical and Medtronic. The other authors have no conflicts of interest to declare.

