Abstract
In patients presenting with a ruptured abdominal aortic aneurysms (AAA), a choice can be made whether or not to offer treatment (selective treatment policy). Patients with a realistic expectation of survival after surgery, identified by several available prediction models, can be offered two treatment options: conventional “open” surgical repair and endovascular “minimally invasive” repair. Conventional open repair carries a significant morbidity and mortality, due to the combined effects of general anaesthesia and surgical exposure. Based on anatomical criteria assessed on a pre-operative CT angiography scan, approximately half of the ruptured AAA are suitable for endovascular aneurysm repair (EVAR). The majority of comparative studies show a clear trend towards lower perioperative mortality for endovascular repair compared to open surgery. The overall analyses of EVAR compared to open surgery, taking one randomised controlled trial and 23 available observational studies into account, showed a 38% decrease in 30-day or hospital mortality rate (Peto odds ratio 0.62; 95% CI 0.52 to 0.74). However, these mainly observational studies show considerable heterogeneity. Furthermore, potential selection bias, selecting patients for endovascular repair constituting a haemodynamically lower-risk category with a more favourable EVAR suitable anatomic configuration, makes a proper comparison unlikely. Therefore, randomised controlled trials, although difficult to perform in an acute severe condition like ruptured AAA, are needed to identify possible benefits of EVAR over open surgery in patients with a ruptured AAA.
Introduction
The incidence of abdominal aortic aneurysms (AAA) has persistently increased over the past few decades1. This is partly attributed to the increased ageing of the population, improved diagnostic tools and the introduction of screening programmes2. To date, AAA are responsible for 1.3% of all deaths among men aged between 65-85 years in developed countries2. This percentage is probably even higher due to the underestimation of AAA-related mortality, since AAA generally exist without symptoms3.
In patients with an identified AAA and abdominal and/or back pain in combination with pain at palpation of the aneurysm (a so-called symptomatic AAA), pending rupture of the AAA is assumed. However, evidence for a symptomatic AAA representing pending rupture is lacking4. When rupture occurs, the mortality rate is as high as 80%5-7. Forty percent of the patients with a ruptured AAA do not reach the hospital alive7 and in patients reaching the hospital and undergoing surgery, the mortality rate is approximately 50%8. Despite progression in surgical techniques, anaesthetical management, vascular prostheses and perioperative care, there has only been a gradual decline in operative mortality rate over the past few decades9,10.
In 1991, a new minimally invasive technique was described by Parodi et al to treat AAA, endovascular aneurysm repair (EVAR)11. In the elective setting, EVAR showed an absolute and relative mortality risk reduction of approximately three and 75%, respectively12,13. In the acute setting, emergency EVAR (eEVAR) is a strategy that might allow for improvement in the above-mentioned poor prognosis. Since 1994 an increasing amount of literature on eEVAR to treat acute AAA has been published. Currently, emergency endovascular aneurysm repair (eEVAR) has become an accepted treatment option, which is increasingly being performed to treat acute AAA. However, the potential reduction in perioperative mortality of eEVAR compared to conventional open repair in patients with an acute AAA is still open to debate.
In this report, we will discuss the role of endovascular AAA repair in patients with a ruptured AAA.
Treatment options
In patients presenting with a ruptured AAA, a choice can be made whether or not to offer treatment at all (selective treatment policy). When it has been decided to perform an intervention, two treatment options are available: conventional “open” AAA repair or the minimally invasive endovascular aneurysm repair (EVAR).
No intervention
In order to identify patients with an unrealistic expectation of a successful outcome after surgery, operative risk predictors, comorbidities and estimated quality of life can be assessed. However, excluding selected patients from treatment is an awkward consideration14,15, hence the number of prediction models generated for risk stratification to support improvement of patient selection for surgical intervention14,16,17. The “Hardman Index” and “Glasgow Aneurysm Score” are the most commonly used prognostic scoring systems. The Hardman Index identifies five independent preoperative factors associated with mortality: age, blood creatinine level, loss of consciousness after arrival, blood haemoglobin level and electrocardiographic ischaemia14. The Glasgow Aneurysm Score uses the following factors: age, shock, myocardial disease, cerebrovascular disease and renal disease17. The validity of both scoring systems was assessed using 82 patients in the study of Tambyraja et al in 200518. Unfortunately, both scoring systems seemed to be poor predictors for postoperative mortality in patients with a ruptured AAA. Two years later, Tambyraja et al identified three risk factors that might form the basis of a new scoring system to predict the outcome of ruptured abdominal aortic aneurysms (rAAA), the “Edinburgh Ruptured Aneurysm Score”19. Risk factors were: blood haemoglobin level, blood pressure, and Glasgow Coma Scale. Until now validation studies are still needed in order to assess its predictive value and clinical applicability.
Due to the modest validity and clinical applicability of present prognostic scoring systems, selecting patients for intervention remains a subjective consideration. Whenever possible, patients’ and families’ opinions, as well as the opinion of the responsible medical doctor have to be included in the decision.
Conventional “open” ruptured AAA repair
Conventional open repair of an AAA was performed for the first time in 1951, replacing the abdominal aortic aneurysm by a homograft20. Two years later, open repair was performed using synthetic grafts21. The open procedure to treat ruptured, as well as, unruptured AAA has been almost consistent over time and known as being an invasive, but generally durable procedure. In patients who often suffer from considerable hypovolaemic shock, a laparotomy is performed immediately after induction of general anaesthesia. Subsequently, the aorta and/or iliacal arteries are clamped proximally and distally from the aneurysm. After clamping, the aneurysm is opened in order to provide access for placement of apolyester tube or bifurcated graft. The aneurysm sac is left in situ and secured around the graft in order to cover it.
This major operation carries a significant mortality and morbidity, due to the combined effects of general anaesthesia, surgical exposure, haemorrhage, and aortic clamping with related lower torso ischaemia-reperfusion injury22. General anaesthesia is required, which might lead to acute haemodynamic changes as aresult of associated inhibition of sympathetic arterial tone. Hypotension and subsequent inadequate oxygenation might induce or accelerate cerebral and/or cardiac ischaemia, resulting in a poor clinical prognosis. Furthermore, loss of abdominal muscle tone can occur during the induction of general anaesthesia, which might cause free rupture of the retroperitoneal haematoma, with related haemodynamic consequences23. During surgical exposure, blood loss is generally extensive24. Hypotension and subsequent inadequate oxygenation might induce or accelerate cerebral and/or cardiac ischaemia, resulting in poor clinical prognosis. Furthermore, after removing the clamps, considerable ischaemia-reperfusion injury of the lower extremities and the intra-abdominal organs might occur.25
Minimally invasive endovascular ruptured AAA repair
In 1991, Parodi et al described a less invasive alternative to conventional “open” aneurysm repair for the treatment of AAA, endovascular aneurysm repair (EVAR)11. EVAR involves groin incisions in order to expose the femoral arteries. Using a catheter and guidewire, a synthetic stent graft is fed through the artery up to the AAA neck until positioned correctly just below the renal arteries and subsequently unfolded, excluding the aneurysm sac from blood flow and pressure. Control angiography is performed to assure correct placement of the endovascular stent graft. Aorto-uni-iliac stent grafts, which reach one of the common iliac arteries as well as bifurcated stent grafts, which reach both iliac arteries, are available. In case of aorto-uni-iliac stent grafting, femoro-femoral bypass graft surgery has to be performed in order to restore blood flow to the contralateral leg. A controlateral endovascular occluder is used to stop retrograde bleeding up into the iliac artery and into the aneurysm sac. Due to increasing expertise and continuous improvement of both stent grafts and their delivery systems, increasing success rates and decreasing complications and re-intervention rates are observed26.
After several years of experience in EVAR for unruptured AAA, this technique has gradually extended its indication and is currently used to treat feasible patients with a ruptured AAA27. However, the applicability for EVAR depends on several anatomical and logistic conditions. Anatomical suitability for EVAR is assessed on a preoperative CTA scan and evaluated for infrarenal aortic neck length, neck angulation, and iliac and femoral access arteries that need to be large enough to accommodate the introducer system28. Approximately half of the ruptured AAA are considered anatomically suitable for eEVAR according to the preoperative CTA scan29. However, logistic problems are often reported that frequently lead to the exclusion of EVAR-suitable patients from undergoing endovascular repair28,30-35. Logistic criteria for EVAR in patients with aruptured AAA are the instant availability of a CT-scanner, the 24/7 availability of an operating room that is adequately equipped to perform endovascular procedures as well as an endovascular trained staff. The availability of a large variety of “off-the-shelf” stent grafts can sometimes lead to high costs36.
EVAR versus open surgery
In a recent systematic review of 61 controlled and uncontrolled clinical studies of patients with an unruptured AAA, EVAR is described as a feasible and safe technique, showing decreased mortality and morbidity rates compared to the conventional open procedure37. Considering these benefits, EVAR has been generally accepted as the preferred treatment option.
Since its first description in 1994 by Yusuf et al27, over 400 reports of EVAR for patients with a ruptured AAA are available. The minimal invasive approach implies the opportunity to use local anaesthesia, which has been proven to be feasible and effective in EVAR38,39. As described by Lachat et al in 2002, local anaesthesia is not attended with the acute haemodynamical changes which are normally seen during induction of general anaesthesia23. However, these benefits have not led to standard application of local anaesthesia, since 19 comparative observational studies show considerable variation in the percentages of patients undergoing local anaesthesia (0-97%). Furthermore, eEVAR involves no cross-clamping and minor surgical exposition compared to open surgery.
The above-mentioned advantageous consequences of the minimally invasive endovascular approach of acute AAA might reflect on perioperative mortality. Approximately 26 studies comparing EVAR with conventional open surgery in patients with a ruptured AAA can be identified28,30-35,40-58. Twenty-four of these studies compared early mortality of EVAR with open surgery28,30-35,40-44,46-53,55-58. One of these studies is a prospective randomised trial by Hinchliffe et al, which showed identical 30-day mortality rates in both treatment groups (9/17 in the open surgery group versus 8/15 in the EVAR group47). However, the study is underpowered and served as a pilot study for future randomised studies. The remaining 23 studies are observational studies of which four showed no reduction in early mortality compared to open surgery47,51,55,56. Using Review Manager 4.2.10, provided by the Nordic Cochrane Centre (part of The Cochrane Collaboration, Oxford, England), a forest plot has been created (Figure1). The overall effect of EVAR compared to open surgery, taking one randomised controlled trial and 23 available observational studies into account, showed a 38% decrease in 30-day or hospital mortality rate (Peto odds ratio 0.62; 95% CI 0.52 to 0.74).
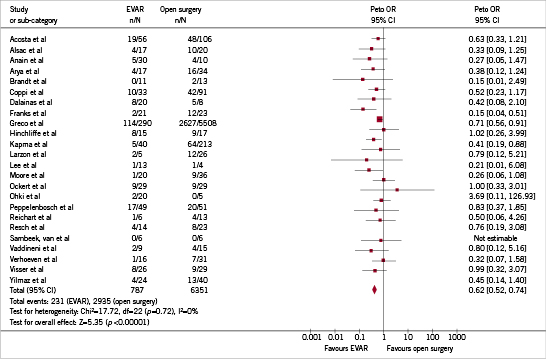
Figure 1. Forest plot of 30-day or hospital mortality in 24 studies comparing EVAR and open surgery in patients with a ruptured AAA.
Additionally, the 30 day, or hospital mortality is reported in five recent systematic reviews (Table 1)24,59-62. Two reviews only discuss the results of the endovascular procedure60,61 and three reviews compare the endovascular with the open procedure24,59,62. The first review showed a pooled mortality rate after EVAR of 24% (95% CI 20-28%) across 31 studies concerning 982 patients61. In 18 observational studies describing 436 people who underwent EVAR, the second review found a pooled mortality of 21% (95% CI 13-29%)60. According to two reviews comparing both treatment groups, pooled mortality is 18%59 and 22%62 in the EVAR group compared to 34%59 and 38%62 in the open surgery group. In the fifth review, Sadat et al showed that EVAR is associated with a significant reduction in mortality with a pooled odds ratio of 0.62 (95% CI 0.52-0.75)24. Visser et al found similar results with an odds ratio of 0.45 (95% BI 0.28-0.72)62. However, after adjustment for patients’ haemodynamic condition, the odds ratio was 0.67 (95% CI 0.31-1.44) and therefore no longer significant.
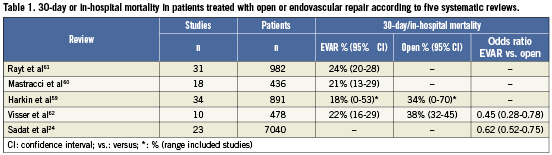
In addition, the systematic reviews showed that EVAR is associated with significant reduction in blood loss, reduced procedure time, reduction in systemical complications and reduced intensive care and hospital stay compared to open surgery24,59,60,62.
Discussion
Theoretically, both the endovascular and the conventional open technique have benefits. On the one hand, during open repair the aorta is clamped short after the initiation of the procedure, ceasing the blood loss. During endovascular repair on the other hand, the ruptured aneurysm remains part of the circulation until the entire endograft is deployed and correctly positioned without major endoleak.
Reported results of reduced early mortality after EVAR for the treatment of a ruptured AAA compared to open surgery seems conclusive (Table1). However, the currently available, mainly observational, studies are small and add considerable heterogeneity and methodological limitations28,30-35,40,42-44,47-49,51-53,63,64. Heterogeneity is signified by the broad range in percentages of patients treated with EVAR (15-50%) and in the percentage of haemodynamically unstable patients (33-73% in the eEVAR group). Even the definition of haemodynamical instability varied between the studies from a systolic blood pressure below 50mmHg to 100mmHg. Furthermore, the comparative studies reported so far are flawed by methodological inadequacies such as high potential of selection bias and lack of randomisation22. Selection bias is created by selecting patients for EVAR constituting a lower-risk category, presuming they need to be haemodynamically more stable for preoperative imaging and have a more favourable (EVAR-suitable) anatomic configuration. In a previous report, though not randomised, we eliminated selection bias due to inadequate patient matching by reporting a comparison of EVAR and open surgery in patients who all had the same preoperative imaging protocol, irrespective of haemodynamic condition, and who were all anatomically suitable for EVAR65. This study showed a significant reduction in 30-day and six-month mortality of EVAR compared to open ruptured AAA repair. However, alarger conducted prospective randomised trial such as the Amsterdam Acute Aneurysm Trial, which is currently being performed in The Netherlands, is needed to identify possible benefits of EVAR over open surgery in patients with a ruptured AAA. The pilot study of Hinchliffe et al showed the possibility to recruit patients with a ruptured AAA to a randomised trial of open surgery and EVAR47. However, a randomised controlled trial (RCT) might give ethical concerns, given the accumulation of superior results with EVAR based on the available observational studies. In addition, aRCT in an acute, severe condition like a ruptured AAA, appears to be difficult to perform47. Furthermore, long-term effects on outcome still need further investigation.
If randomised trials demonstrate a clinically relevant reduction in mortality and morbidity for endovascular repair, consequences for care organisation will be major. Treatment of ruptured AAA has to be performed in hospitals that are able to guarantee permanent availability of endovascular trained staff, implicating regionalisation and centralisation of acute AAA care.
Conclusion
The minimally invasive endovascular procedure (EVAR) is theoretically likely to reduce early mortality in patients with a ruptured AAA. The majority of observational studies show a clear trend toward an improved short-term effect of EVAR and a significant reduction in early mortality compared to conventional open surgery. Therefore, EVAR has become a generally accepted treatment option for ruptured AAA. However, studies comparing EVAR with conventional open surgery have to be interpreted with caution due to the likelihood of methodological inadequacies such as selection bias, heterogeneity, and lack of randomisation. Can endovascular repair of the ruptured AAA be considered as the treatment option of first choice? This question has not been answered yet. Further research in terms of randomised controlled trials with adequate follow-up will be required in order to clarify the role of endovascular repair as a treatment option for ruptured abdominal aortic aneurysms.
Conflict of interest statement
The authors have no conflicts of interest to declare.

