
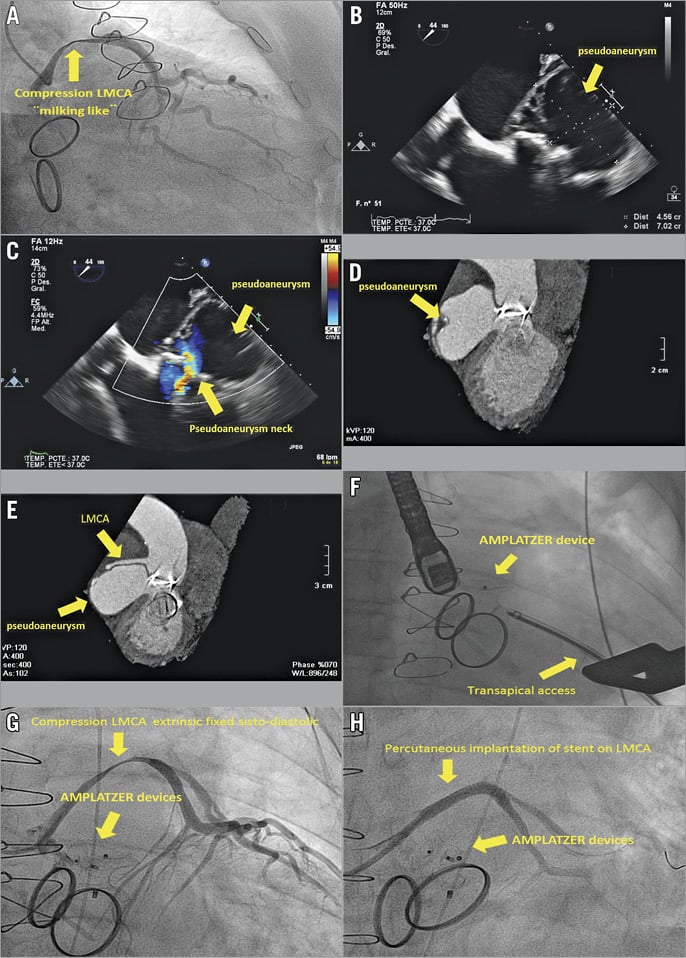
An 83-year-old male patient who had had mitral-aortic valve replacement in 1998 due to native valve infective endocarditis was admitted for elective coronary angiography after he had shown left ventricular systolic dysfunction with prosthetic normal function during his ambulatory control. The study showed severe extrinsic systolic compression in the left main coronary artery (LMCA), with a “milking effect” and a fistula image draining into a cavity of inferoposterior location (Panel A, Moving image 1).
Transoesophageal echocardiography (TEE) showed a 70×40 mm pseudoaneurysm of the mitral-aortic intervalvular fibrosa, with fistula formation in the outflow tract of the left ventricle (LV) (Panel B, Panel C, Moving image 2-Moving image 5). Cardiac computed tomography (CT) was also performed (Panel D, Panel E). Active endocarditis was excluded by clinic analytics and positron emission tomography/computed tomography (PET-CT). Surgery was ruled out in this patient due to high surgical risk (EuroSCORE 25, 93%), and therefore a percutaneous treatment was planned. Intervention was carried out through transapical access and the closure was made with AMPLATZER™ devices (AMPLATZER™ Muscular VSD Occluder, 18 mm, AMPLATZER™ Duct Occluder, 10/8 mm; St. Jude Medical, St. Paul, MN, USA) in the pseudoaneurysm necks (Panel F, Moving image 6).
Due to the risk of bleeding following dual antiplatelet therapy at the time of performing the transapical access, stenting of the LMCA was postponed. During transfer to the ICU, the patient showed severe hypotension with QRS widening. Urgent coronary angiography was performed, which showed extrinsic fixed compression of the LMCA (Panel G, Moving image 7). Prosthetic valve function was normal under fluoroscopy. Two drug-eluting stents were implanted and, due to a persistent fistula, a PK Papyrus stent (Biotronik, Berlin, Germany) was finally implanted (Panel H, Moving image 8). Throughout the procedure, the patient presented cardiac arrest correctly reanimated. Later, the patient was admitted to the ICU with cardiogenic shock, which finally resulted in his death.
Extrinsic compression of the LMCA is an infrequent pathology, clinically manifest by coronary ischaemia1. The main cause depicted in the literature is the dilation of the pulmonary artery2.
A milking effect by external compression is an unusual finding in coronary angiography. Ferrer-Hita et al3 reported a case of systolic ventricular pseudoaneurysm “milking-like” compression of the posterolateral artery. LV dysfunction also represents a clinical expression of ischaemia in this situation4.
In this patient, the pseudoaneurysm was probably a low-pressure cavity during diastole. After closing the main entrance, the intracavitary pressure may have risen above the systemic, causing the fixed compression of the LMCA leading to cardiogenic shock. The stent implant over the LMCA should probably have preceded the percutaneous solution of the pseudoaneurysm closure. However, the question is when and how this could have been done, given the mitral-aortic prosthesis and that transapical entry was the only possible access.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Coronary angiography. “Milking-like” compression of the LMCA.
Moving image 2. Transoesophageal echocardiography. TEE to 44º. Pseudoaneurysm.
Moving image 3. Transoesophageal echocardiography. TEE x plane imaging. Pseudoaneurysm.
Moving image 4. Transoesophageal echocardiography 3D. Pseudoaneurysm.
Moving image 5. Transoesophageal echocardiography. TEE to 44º. Pseudoaneurysm neck. Colour flow there through.
Moving image 6. Coronary angiography. Percutaneous implantation of the AMPLATZER device in the neck of the pseudoaneurysm.
Moving image 7. Coronary angiography. Fixed systolic-diastolic compression due to pseudoaneurysm after implanting device.
Moving image 8. Coronary angiography. Percutaneous implantation of a stent on the LMCA.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Coronary angiography. “Milking-like” compression of the LMCA.
Moving image 2. Transoesophageal echocardiography. TEE to 44º. Pseudoaneurysm.
Moving image 3. Transoesophageal echocardiography. TEE x plane imaging. Pseudoaneurysm.
Moving image 4. Transoesophageal echocardiography 3D. Pseudoaneurysm.
Moving image 5. Transoesophageal echocardiography. TEE to 44º. Pseudoaneurysm neck. Colour flow there through.
Moving image 6. Coronary angiography. Percutaneous implantation of the AMPLATZER device in the neck of the pseudoaneurysm.
Moving image 7. Coronary angiography. Fixed systolic-diastolic compression due to pseudoaneurysm after implanting device.
Moving image 8. Coronary angiography. Percutaneous implantation of a stent on the LMCA.

